Subatomic Particles particle symbols Relative electric charge Mass

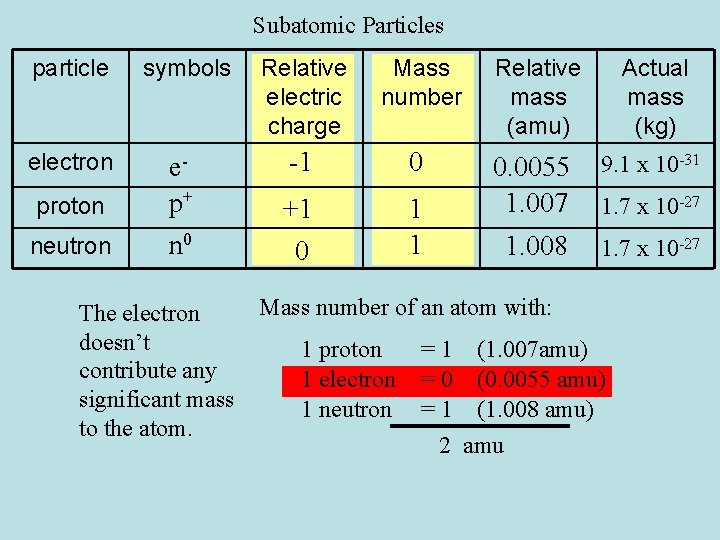
Subatomic Particles particle symbols Relative electric charge Mass number Relative mass (amu) Actual mass (kg) electron ep+ n 0 -1 0 9. 1 x 10 -31 +1 0 1 1 0. 0055 1. 007 1. 008 1. 7 x 10 -27 proton neutron The electron doesn’t contribute any significant mass to the atom. 1. 7 x 10 -27 Mass number of an atom with: 1 proton 1 electron 1 neutron = 1 (1. 007 amu) = 0 (0. 0055 amu) = 1 (1. 008 amu) 2 amu

Atomic number is the number of protons Mass number is the number of protons + neutrons. An atom with 6 protons and 7 neutrons has an atomic number of 6 and a mass number of 13. This atom would be carbon because carbon’s atomic number is 6.

Isotopes are atoms of the same element with different masses. Not all atoms of an element are the same. Not all atoms of hydrogen are the same. There exists 3 isotopes (types) of hydrogen. Substitute the word variety (or flavors).
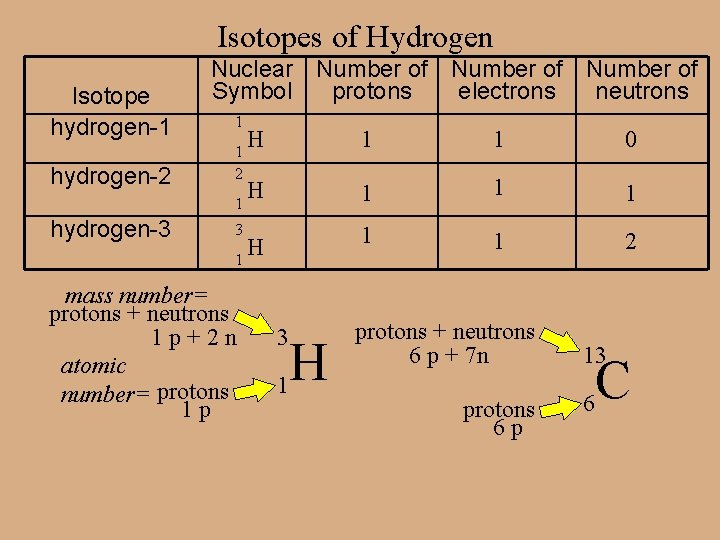
Isotopes of Hydrogen Isotope hydrogen-1 Nuclear Number of Symbol protons electrons neutrons 1 H 1 1 0 hydrogen-2 1 2 H 1 1 hydrogen-3 3 1 1 2 H 1 mass number= protons + neutrons 1 p+2 n atomic number= protons 1 p 3 H 1 protons + neutrons 6 p + 7 n protons 6 p 13 C 6
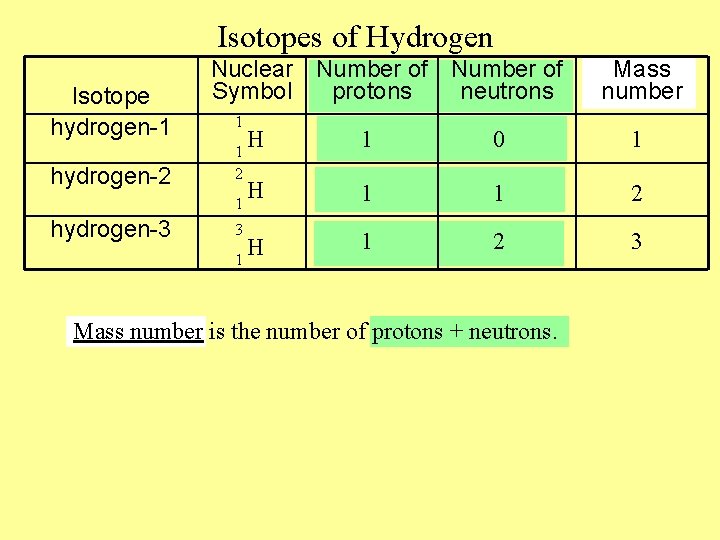
Isotopes of Hydrogen Isotope hydrogen-1 hydrogen-2 Nuclear Number of Symbol protons neutrons 1 H 1 0 1 H 1 1 2 3 1 2 1 hydrogen-3 Mass number is the number of protons + neutrons.
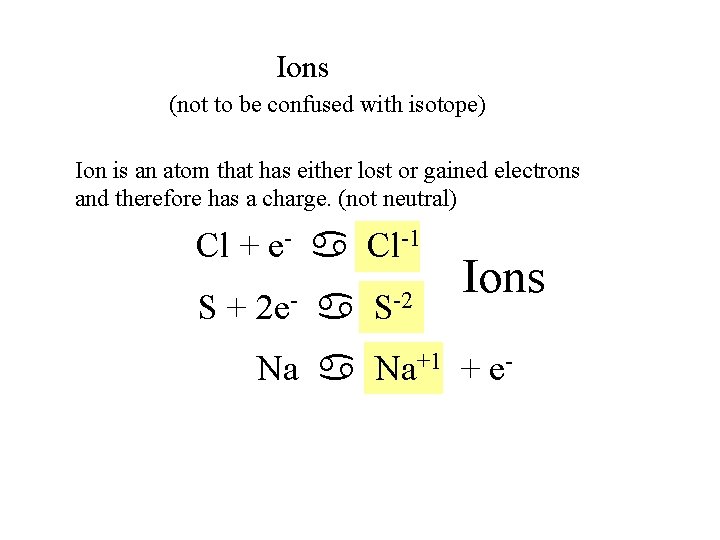
Ions (not to be confused with isotope) Ion is an atom that has either lost or gained electrons and therefore has a charge. (not neutral) Cl + e- a Cl-1 S + 2 e- a S-2 Ions Na a Na+1 + e-
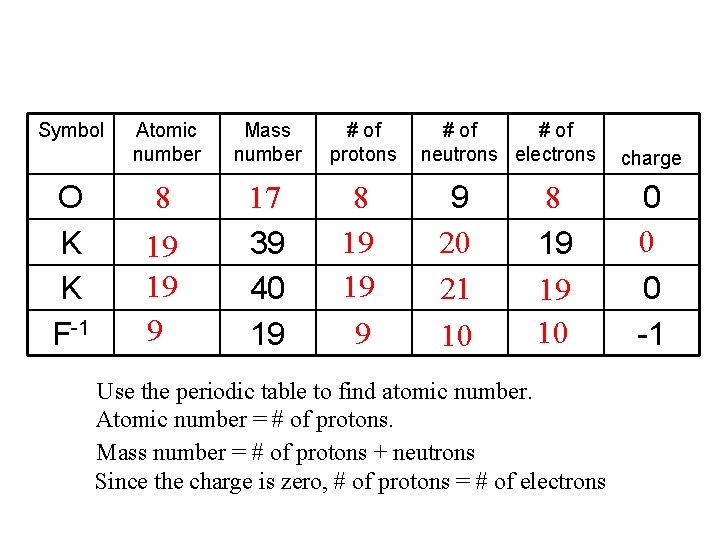
Symbol O K K F-1 Atomic number Mass number # of protons 8 17 39 40 19 8 19 19 9 # of neutrons electrons 9 20 21 10 8 19 19 10 Use the periodic table to find atomic number. Atomic number = # of protons. Mass number = # of protons + neutrons Since the charge is zero, # of protons = # of electrons charge 0 0 0 -1
- Slides: 8