SubAtomic Particles Electron Proton and Neutron J J
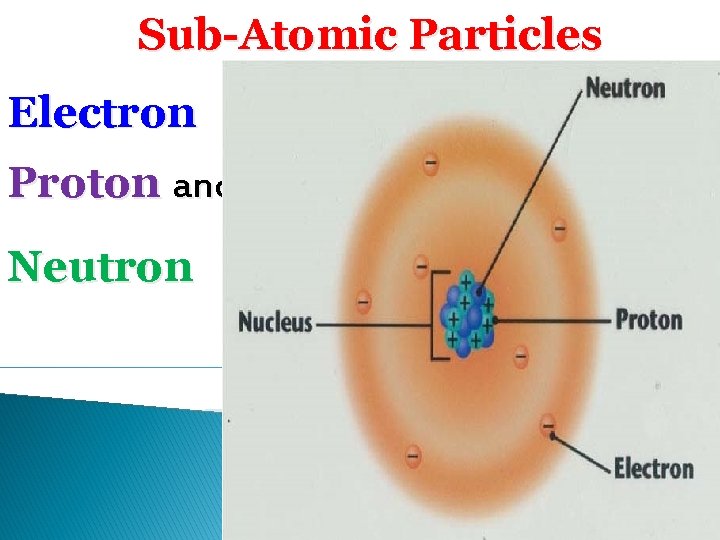
Sub-Atomic Particles Electron Proton and Neutron
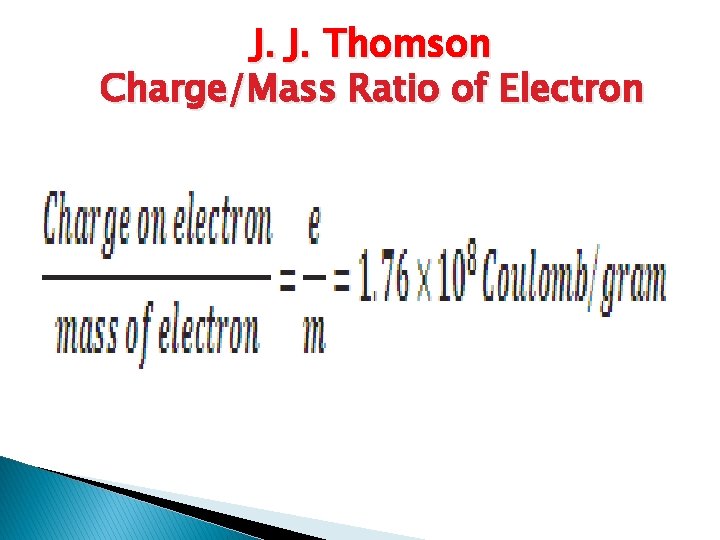
J. J. Thomson Charge/Mass Ratio of Electron
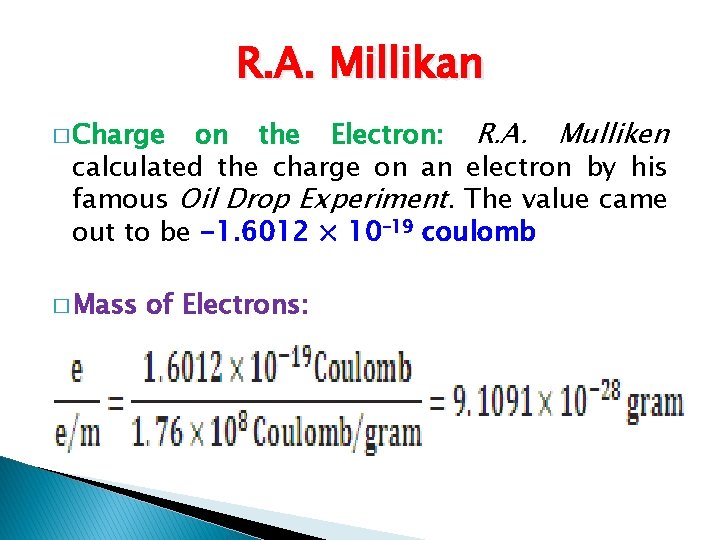
R. A. Millikan on the Electron: R. A. Mulliken calculated the charge on an electron by his famous Oil Drop Experiment. The value came out to be -1. 6012 × 10– 19 coulomb � Charge � Mass of Electrons:
�A Eugene Goldstein dim glow is visible behind the cathode when an electric discharge is passed through a perforated cathode in a discharge tube filled with a gas at low pressure � These new type of rays travel from anode to that cathode � He gave the name canal rays to these rays because these rays cross the canals of the cathode and reach the other side

�W. Wein proved that the canal rays consist of positively charged particles �J. J. Thomson gave the name positive rays to them because they are composed of positively charged particle
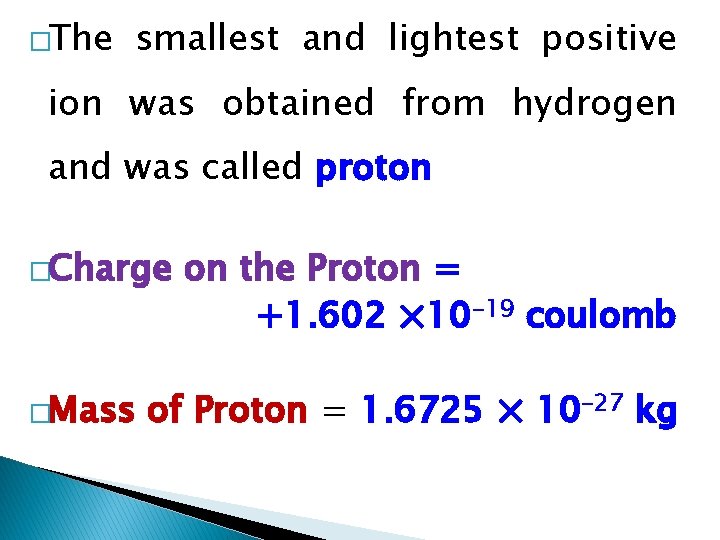
�The smallest and lightest positive ion was obtained from hydrogen and was called proton �Charge �Mass on the Proton = +1. 602 × 10– 19 coulomb of Proton = 1. 6725 × 10– 27 kg
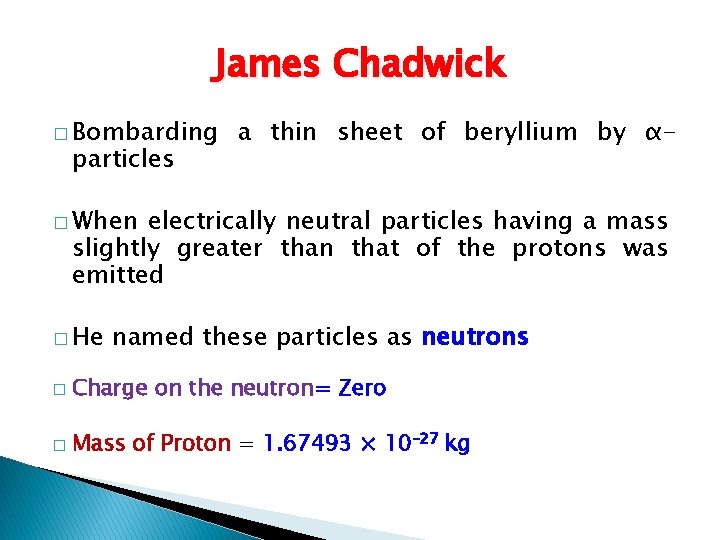
James Chadwick � Bombarding particles a thin sheet of beryllium by α- � When electrically neutral particles having a mass slightly greater than that of the protons was emitted � He named these particles as neutrons � Charge on the neutron= Zero � Mass of Proton = 1. 67493 × 10– 27 kg
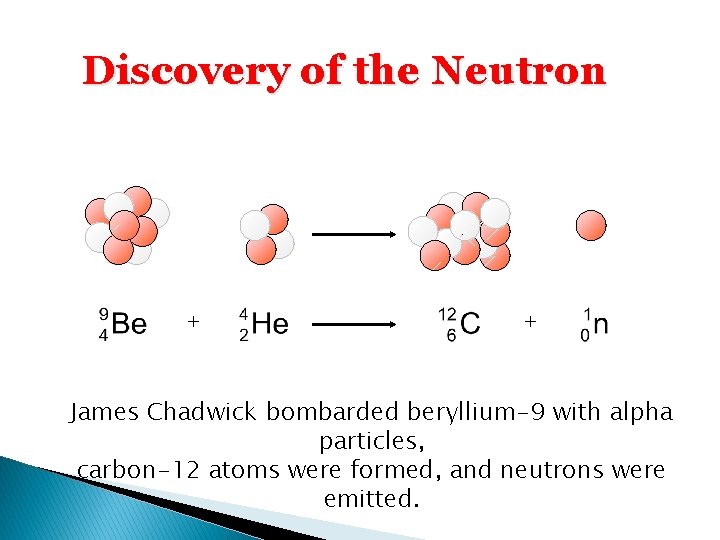
Discovery of the Neutron + + James Chadwick bombarded beryllium-9 with alpha particles, carbon-12 atoms were formed, and neutrons were emitted.
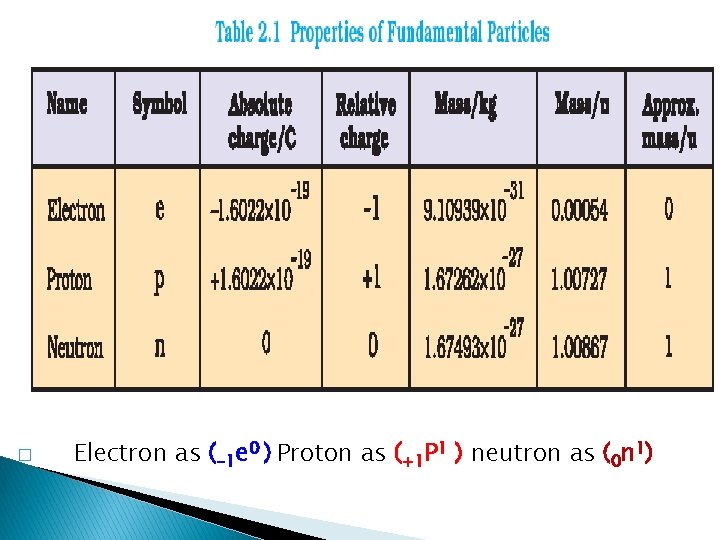
� Electron as (-1 e 0 ) Proton as (+1 P 1 ) neutron as (0 n 1)
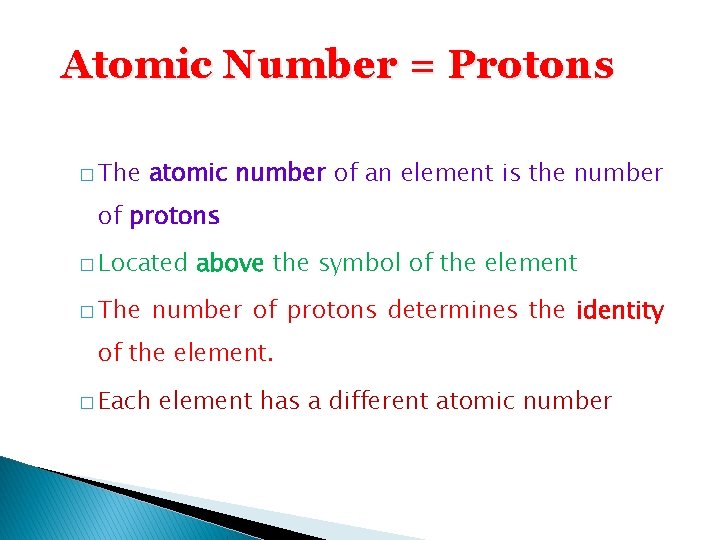
Atomic Number = Protons � The atomic number of an element is the number of protons � Located � The above the symbol of the element number of protons determines the identity of the element. � Each element has a different atomic number
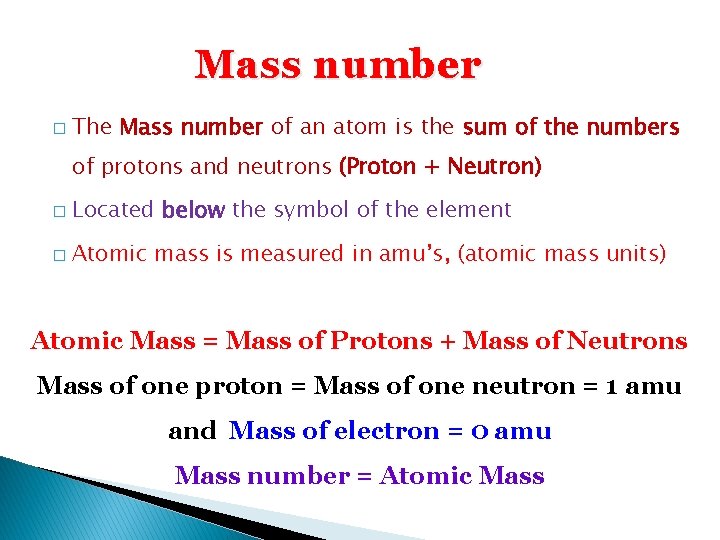
Mass number � The Mass number of an atom is the sum of the numbers of protons and neutrons (Proton + Neutron) � Located below the symbol of the element � Atomic mass is measured in amu’s, (atomic mass units) Atomic Mass = Mass of Protons + Mass of Neutrons Mass of one proton = Mass of one neutron = 1 amu and Mass of electron = 0 amu Mass number = Atomic Mass

How many electrons are in an atom? � For an atom to have an overall neutral charge the number of electrons must equal the number of protons � Protons= electrons
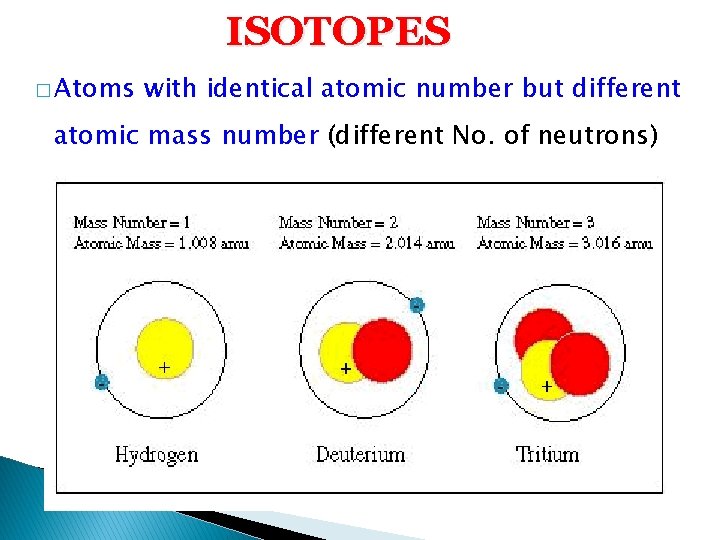
ISOTOPES � Atoms with identical atomic number but different atomic mass number (different No. of neutrons)
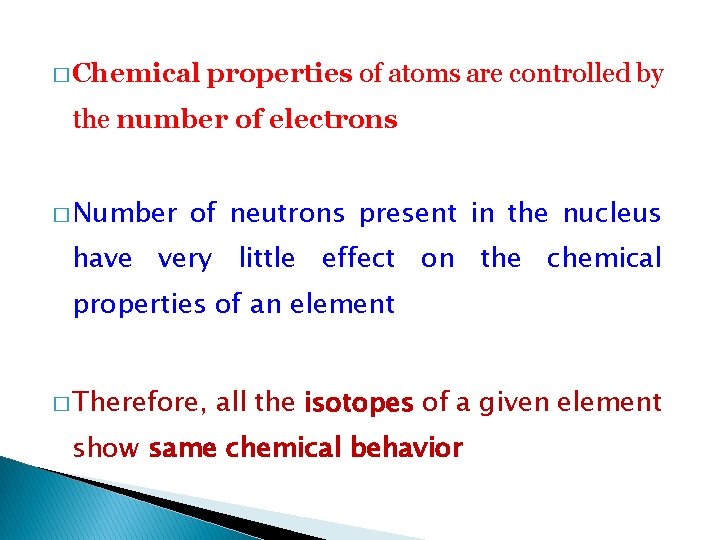
� Chemical properties of atoms are controlled by the number of electrons � Number of neutrons present in the nucleus have very little effect on the chemical properties of an element � Therefore, all the isotopes of a given element show same chemical behavior
Isobars � Isobars are the atoms with same mass number but different atomic number (different No. of protons) � 14 and N 14 C 6 7 � Mass number = Proton + neutron (6+8=14 in C 7+7=14 in N) � Atomic � All Number = protons (6 in C and 7 in N) the isobars of a given element show different chemical behavior

The end
- Slides: 16