Subatomic Particles Electron JJ Thomson discovered the electron

Subatomic Particles

Electron JJ Thomson discovered the electron using cathode ray experiment Electron – smallest particle in an atom Negatively charged Directly involved in chemical reactions Hangs out in the Electron Cloud – area around the nucleus where the electrons exist; mostly empty space; denser in areas where electrons are likely to exist Ion – An atom that gains or loses electrons

Nucleus Rutherford & the Gold Foil Experiment Discovered the nucleus Nucleus – densely packed group of protons and neutrons in the center of the atom Very tiny compared to whole atom Electron cloud occupies most of the volume of the atom
Nucleus – Protons & Neutrons Within the nucleus: Proton - positively charged subatomic particle Very heavy compared to the electron Located in the nucleus Neutron – neutral subatomic particle About the same mass as a proton Adds mass and stability to the nucleus Discovered by Chadwick
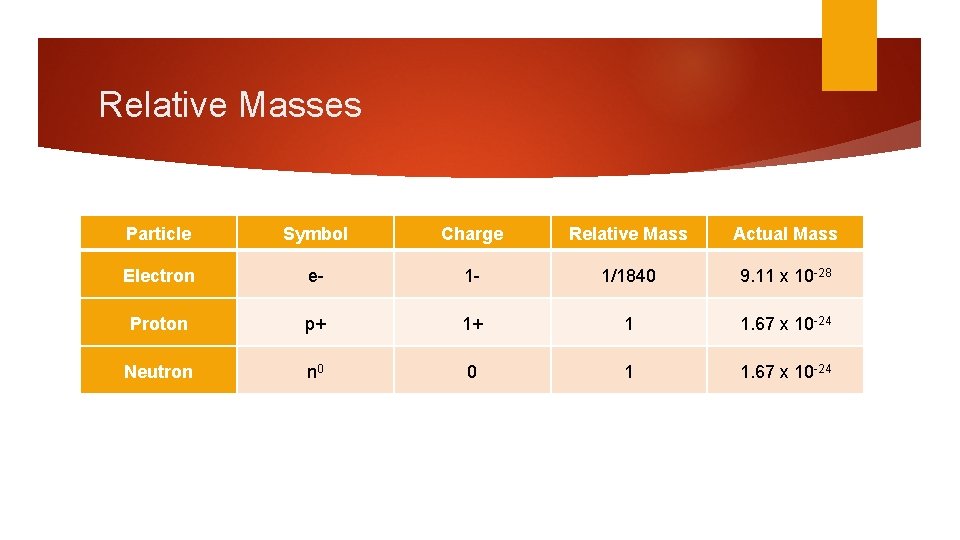
Relative Masses Particle Symbol Charge Relative Mass Actual Mass Electron e- 1 - 1/1840 9. 11 x 10 -28 Proton p+ 1+ 1 1. 67 x 10 -24 Neutron n 0 0 1 1. 67 x 10 -24
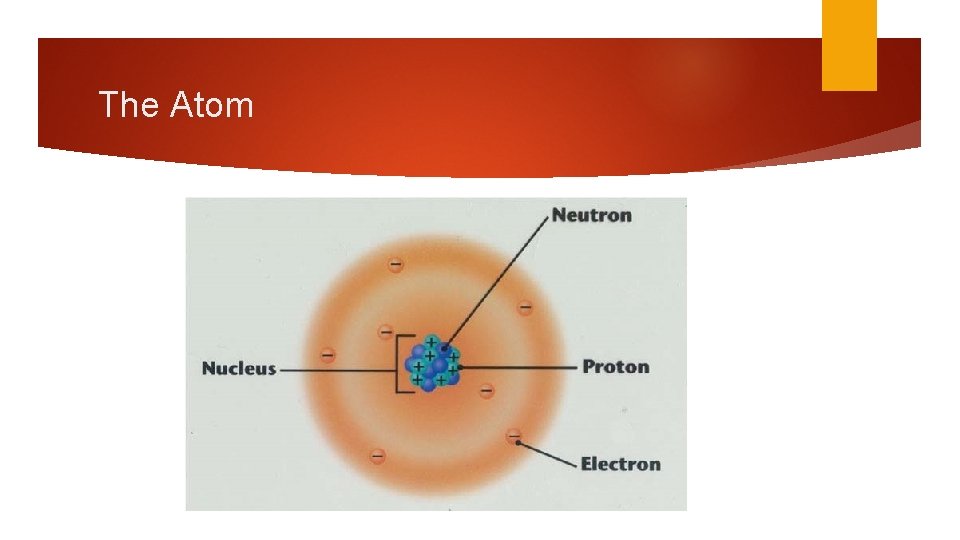
The Atom
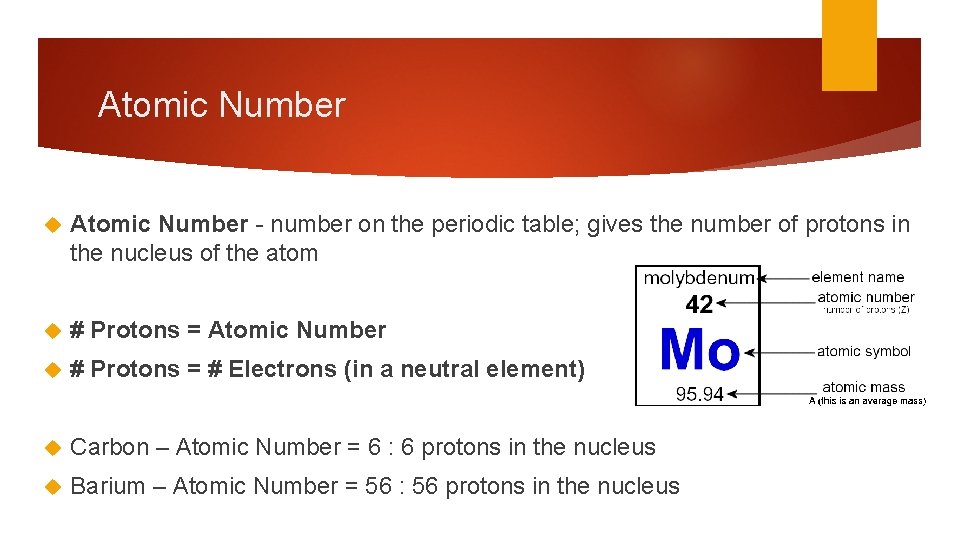
Atomic Number - number on the periodic table; gives the number of protons in the nucleus of the atom # Protons = Atomic Number # Protons = # Electrons (in a neutral element) Carbon – Atomic Number = 6 : 6 protons in the nucleus Barium – Atomic Number = 56 : 56 protons in the nucleus
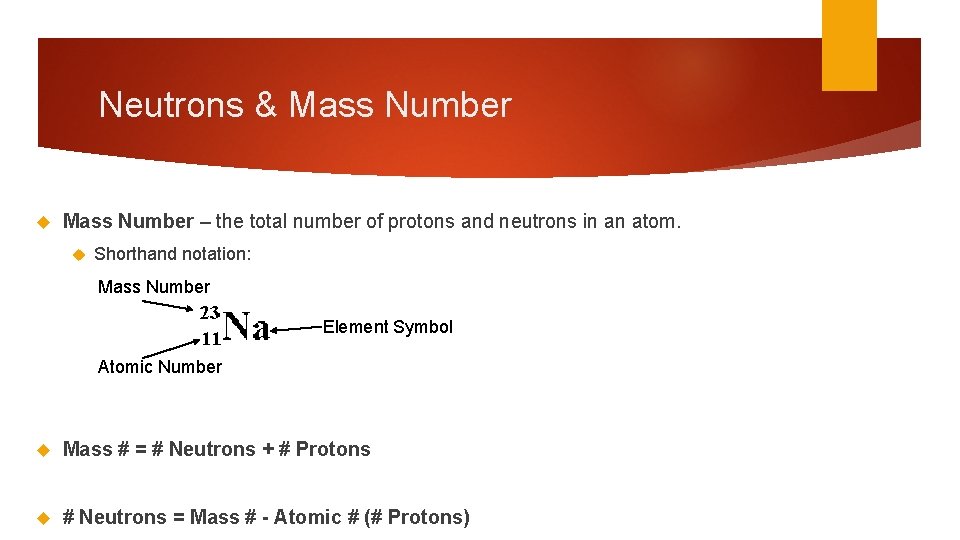
Neutrons & Mass Number – the total number of protons and neutrons in an atom. Shorthand notation: Mass Number Element Symbol Atomic Number Mass # = # Neutrons + # Protons # Neutrons = Mass # - Atomic # (# Protons)
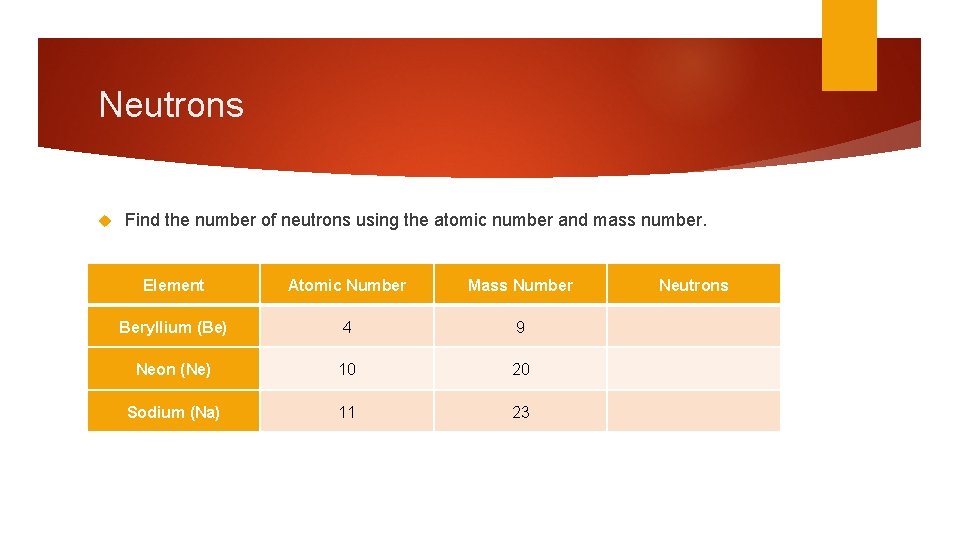
Neutrons Find the number of neutrons using the atomic number and mass number. Element Atomic Number Mass Number Beryllium (Be) 4 9 Neon (Ne) 10 20 Sodium (Na) 11 23 Neutrons

Isotope – An atom that gains or loses a neutron. Same number of protons, but different numbers of neutrons Different neutrons = different masses Same chemical, just a different mass Neon-20 – 10 protons, 10 neutrons, 10 electrons Neon-21 – 10 protons, 11 neutrons, 10 electrons Neon-22 – 10 protons, 12 neutrons, 10 electrons

Atomic Mass AMU – Atomic Mass Unit used for measuring the mass of an atom Atomic Mass Number – weighted average mass of the atoms in a naturally occurring sample of the element. Masses on the periodic table are decimals because they are an AVERAGE of the abundances of the isotopes. Cl-35: 75% Cl-37: 25% Chlorine on the periodic table: 35. 4527 g/mol
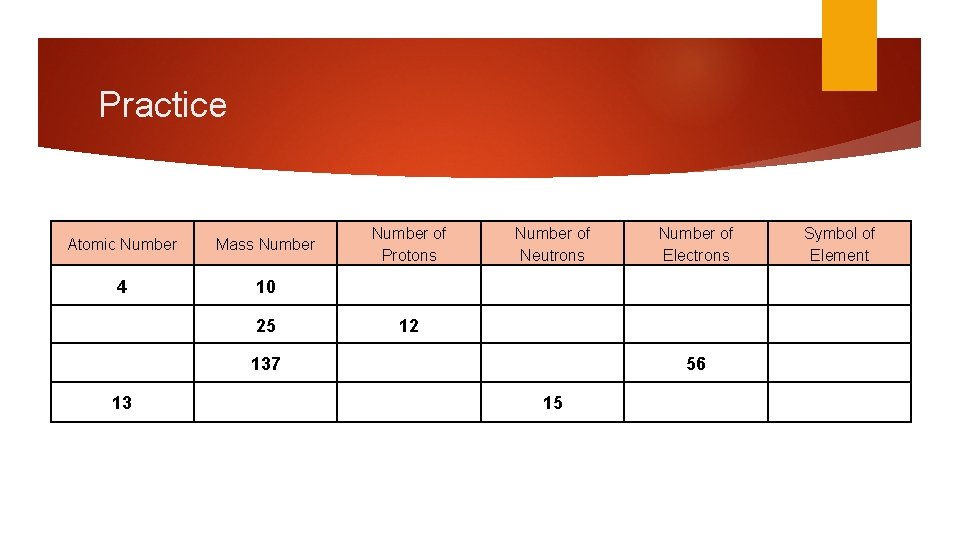
Practice Atomic Number Mass Number 4 10 25 Number of Protons Number of Neutrons 12 137 13 Number of Electrons 56 15 Symbol of Element
- Slides: 12