Subatomic particle Charge Location proton nucleus neutron none

• Subatomic particle Charge Location proton + nucleus neutron none nucleus electron surrounds nucleus Atomic number (#)= number of protons (=number of electrons in neutral atom) Mass number (AM)= number of protons + number of neutrons
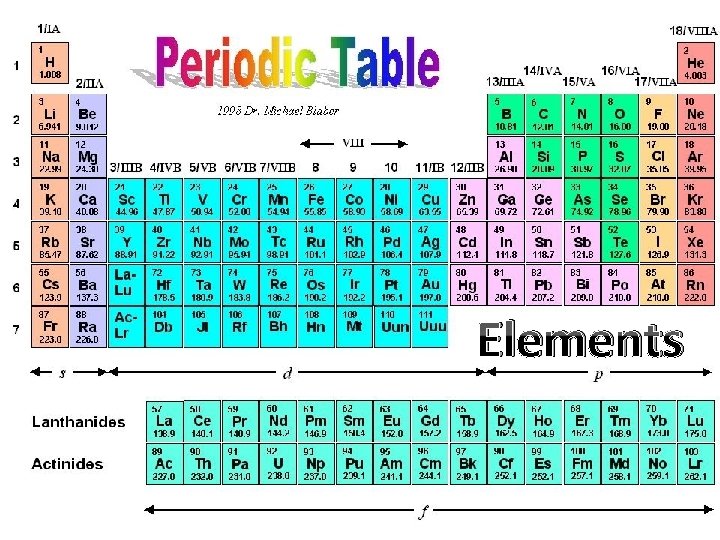
Elements

Macroelements- need large quantities 6 elements make up 98% by mass C, H, N, O, P, S Microelements (or trace elements)Need very small amounts but critical!! ex. Selenium (Se) is an antioxidant that helps prevent cell damage from free radicals, also help thyroid function and boost immune system
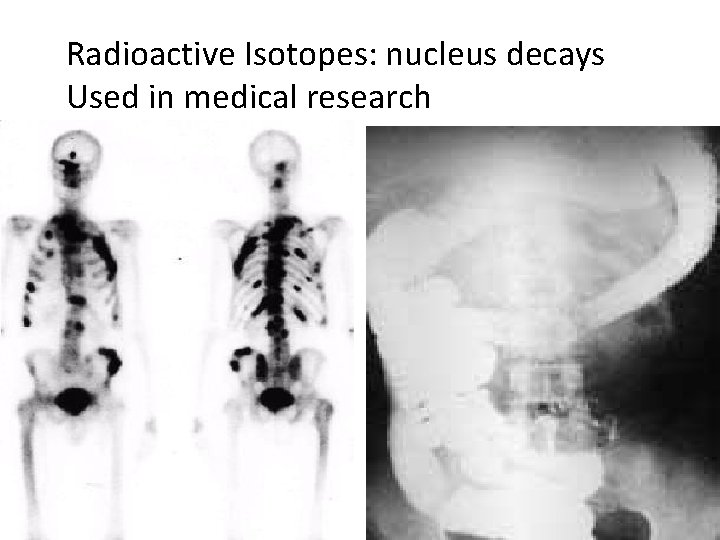
Radioactive Isotopes: nucleus decays Used in medical research
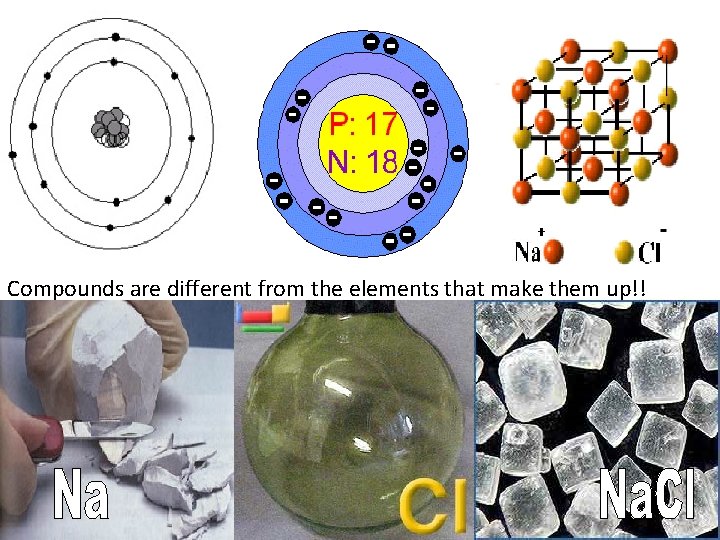
Compounds are different from the elements that make them up!!
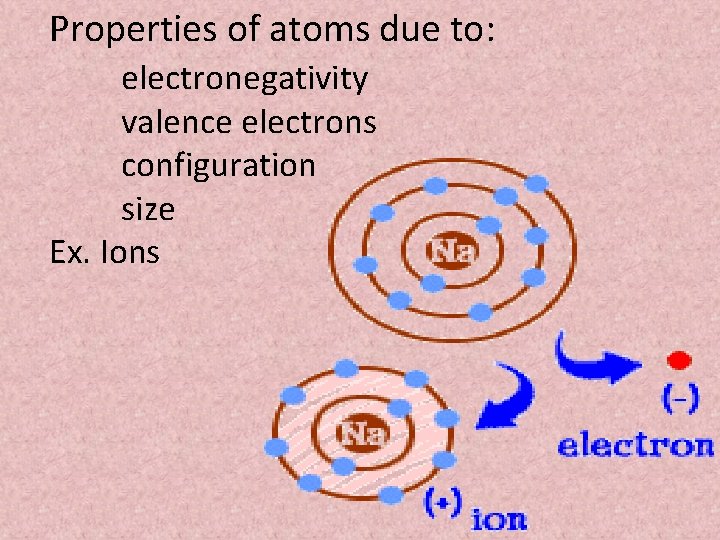
Properties of atoms due to: electronegativity valence electrons configuration size Ex. Ions
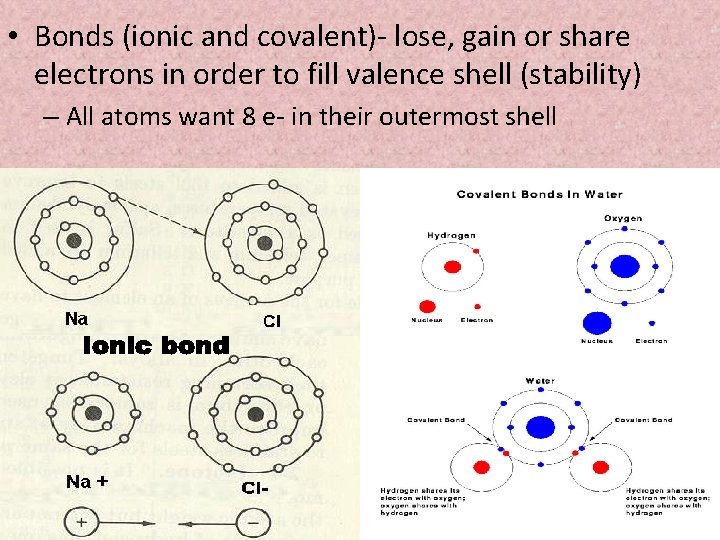
• Bonds (ionic and covalent)- lose, gain or share electrons in order to fill valence shell (stability) – All atoms want 8 e- in their outermost shell
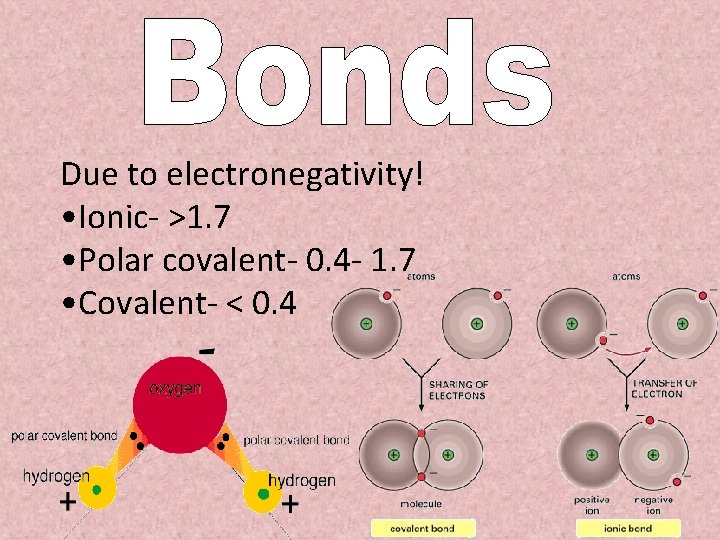
Due to electronegativity! • Ionic- >1. 7 • Polar covalent- 0. 4 - 1. 7 • Covalent- < 0. 4

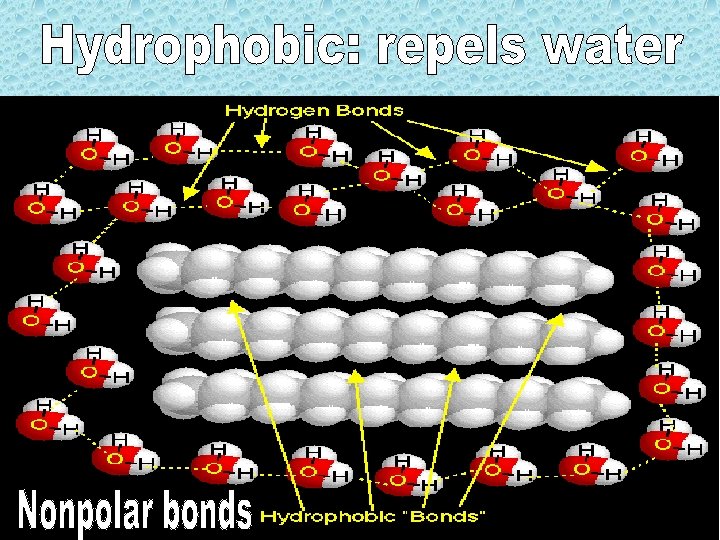
• Hydrogen bondsattraction of H to partial negative charge (due to polar covalent bonds between oxygen and hydrogen)
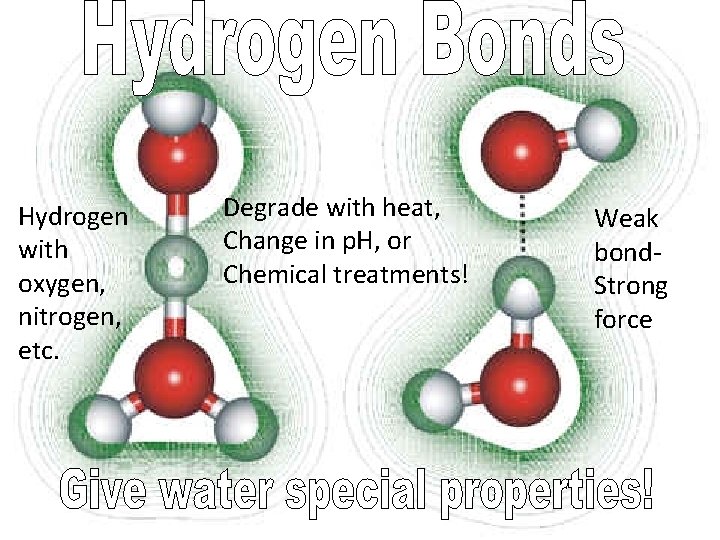
Hydrogen with oxygen, nitrogen, etc. Degrade with heat, Change in p. H, or Chemical treatments! Weak bond. Strong force
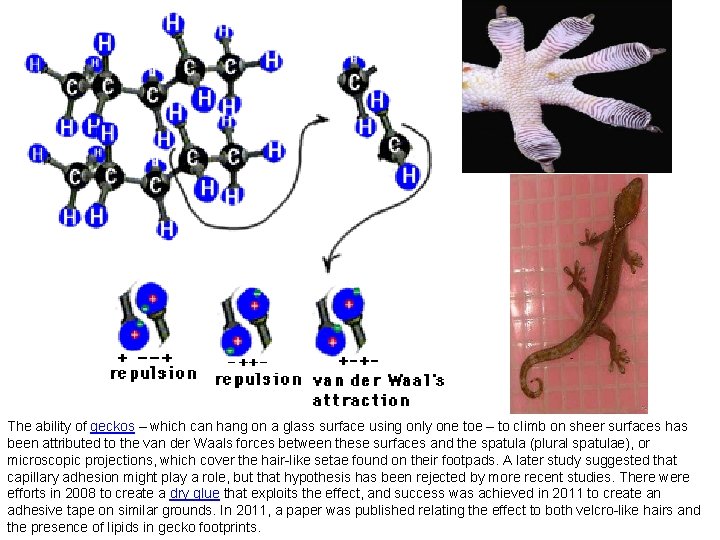
The ability of geckos – which can hang on a glass surface using only one toe – to climb on sheer surfaces has been attributed to the van der Waals forces between these surfaces and the spatula (plural spatulae), or microscopic projections, which cover the hair-like setae found on their footpads. A later study suggested that capillary adhesion might play a role, but that hypothesis has been rejected by more recent studies. There were efforts in 2008 to create a dry glue that exploits the effect, and success was achieved in 2011 to create an adhesive tape on similar grounds. In 2011, a paper was published relating the effect to both velcro-like hairs and the presence of lipids in gecko footprints.
And remember: Structure determines Function!!!!!

Should we control a chemical that: • Causes excessive sweating and vomiting. • Is a major component in acid rain. • Can cause severe burns in its gaseous state. • Accidental inhalation can kill you. • Contributes to erosion. • Decreases the effectiveness of car brakes. • Has been found in tumors of terminal cancer patients.

What is the chemical? Dihydrogen monoxide





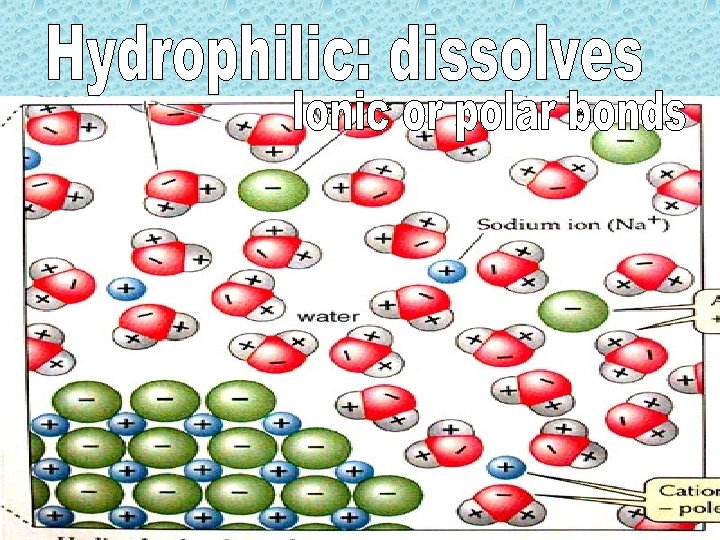
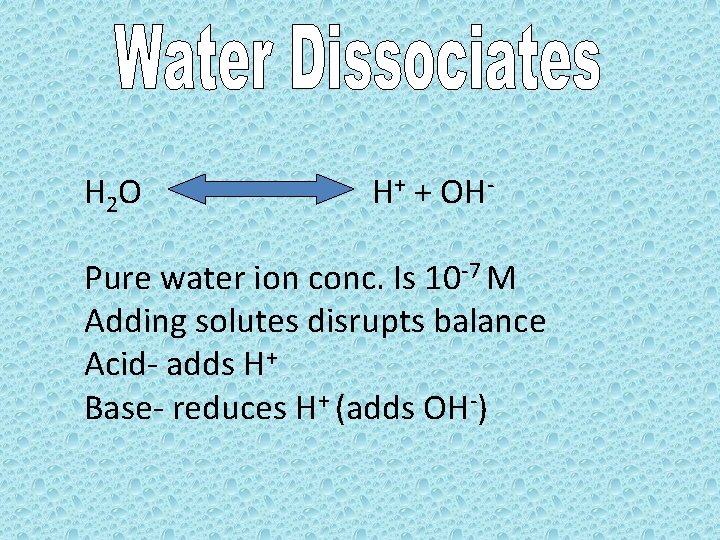
H 2 O H+ + OH- Pure water ion conc. Is 10 -7 M Adding solutes disrupts balance Acid- adds H+ Base- reduces H+ (adds OH-)
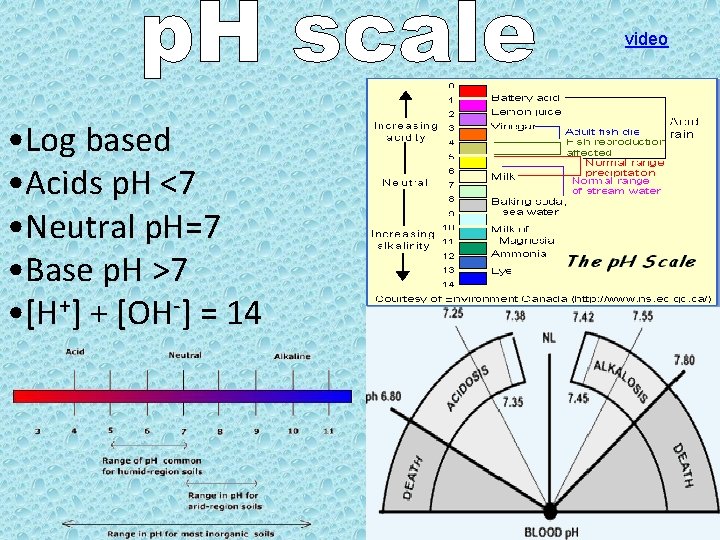
video • Log based • Acids p. H <7 • Neutral p. H=7 • Base p. H >7 • [H+] + [OH-] = 14
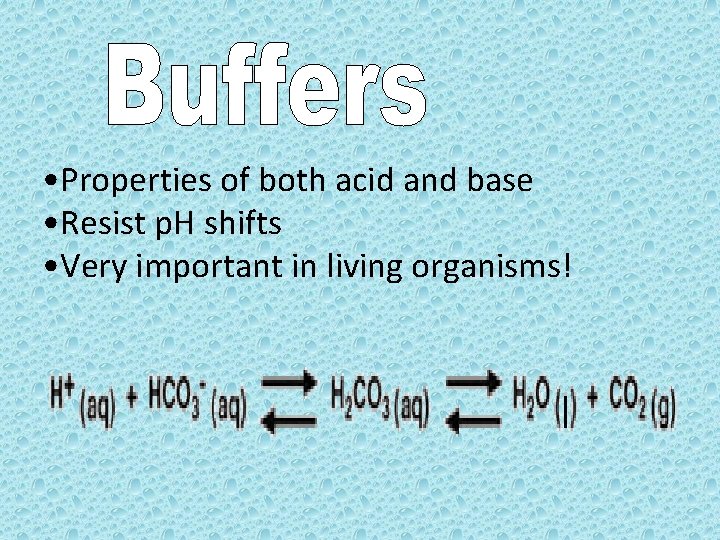
• Properties of both acid and base • Resist p. H shifts • Very important in living organisms!

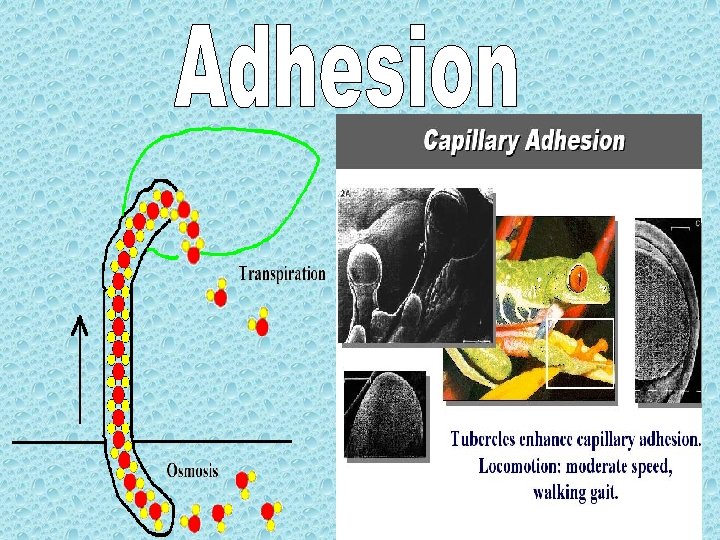
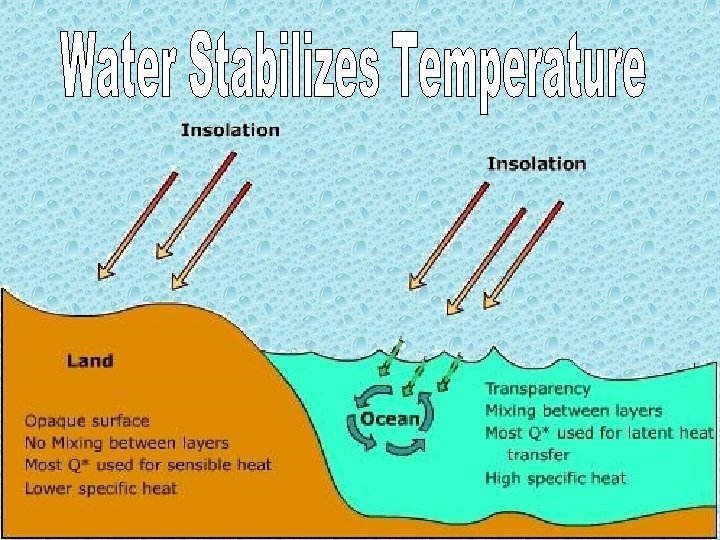
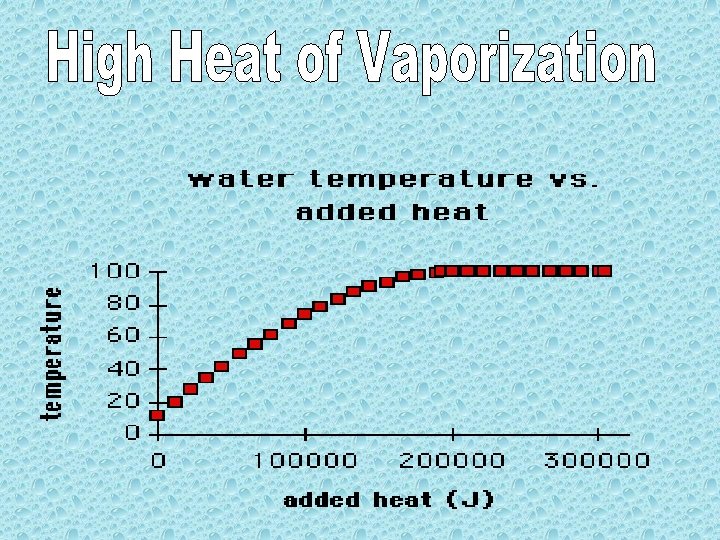
2006 - The movement of water through vascular plants is important to their survival. a. Explain the mechanism of water movement through vascular plants during transpiration. Include a discussion of how the anatomy of vascular plants and the properties of water contribute to this process. 2003 B- Water is important for all living organisms. The functions of water are directly related to its physical properties a. Describe how the properties of water contribute to TWO of the following * transpiration * thermoregulation in endotherms * plasma membrane structure
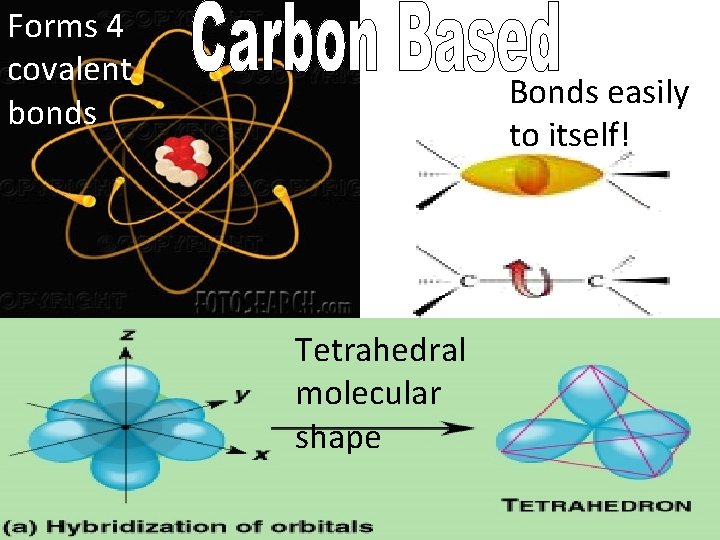
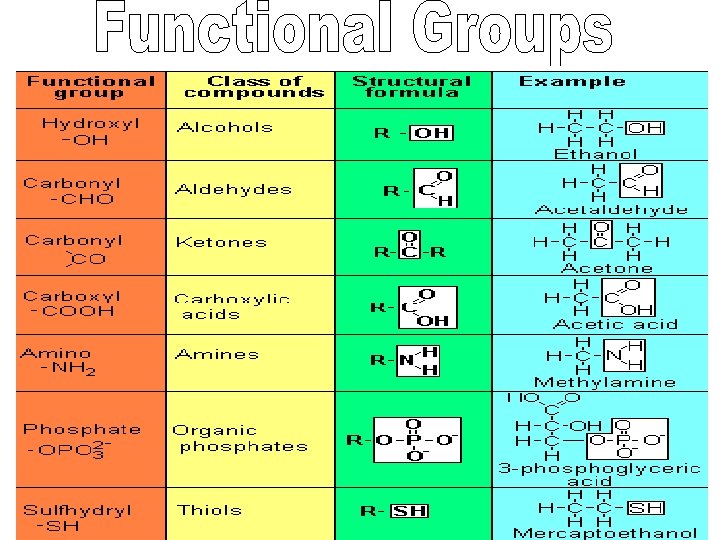
Forms 4 covalent bonds Bonds easily to itself! Tetrahedral molecular shape
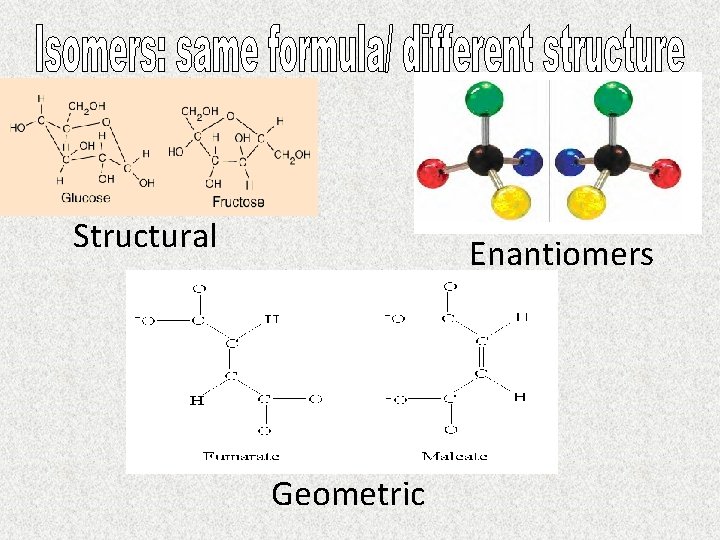
Structural Enantiomers Geometric
Built of monomers (subunits)
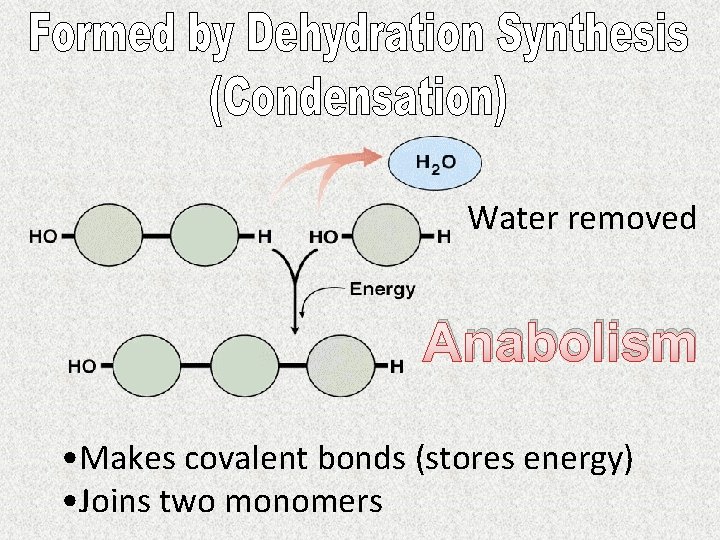
Water removed Anabolism • Makes covalent bonds (stores energy) • Joins two monomers
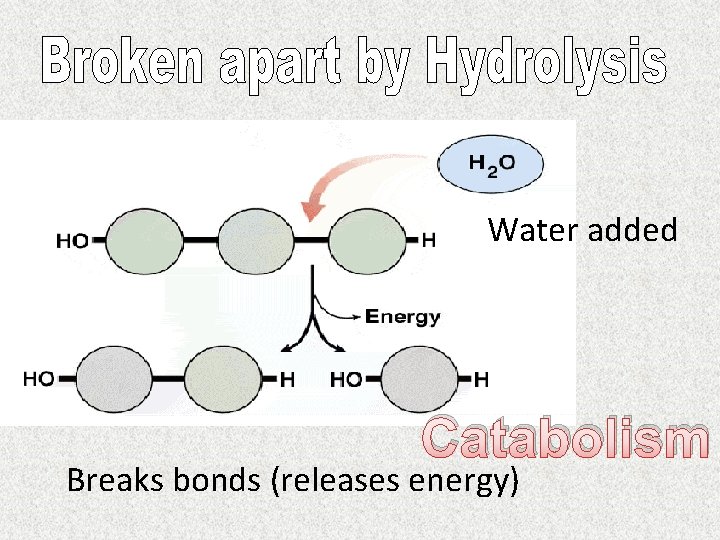
Water added Catabolism Breaks bonds (releases energy)

2003 B- Water is important for all living organisms. The functions of water are directly related to its physical properties. b. Water serves as a reactant and a product in the carbon cycle. Discuss the role of water in the carbon cycle.

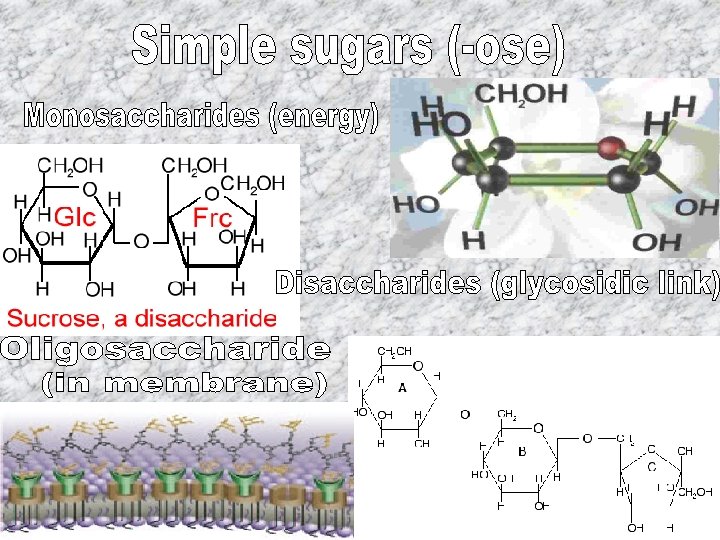
• C, H, O in 1: 2: 1 ratio • Used for fuel, structure, and receptors • Simple or complex
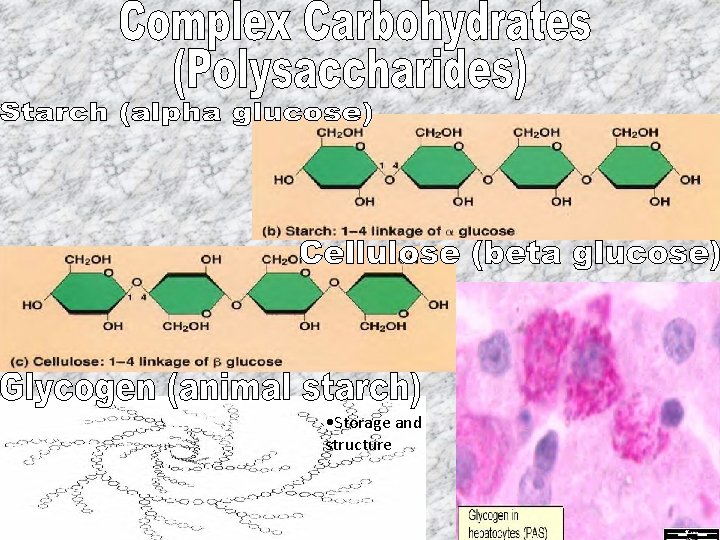
• Storage and structure

Chitin

• Hydrophobic • C, H, O components- high in C • High in energy

• Fats solid, oils liquid • Made of fatty acids and glycerol
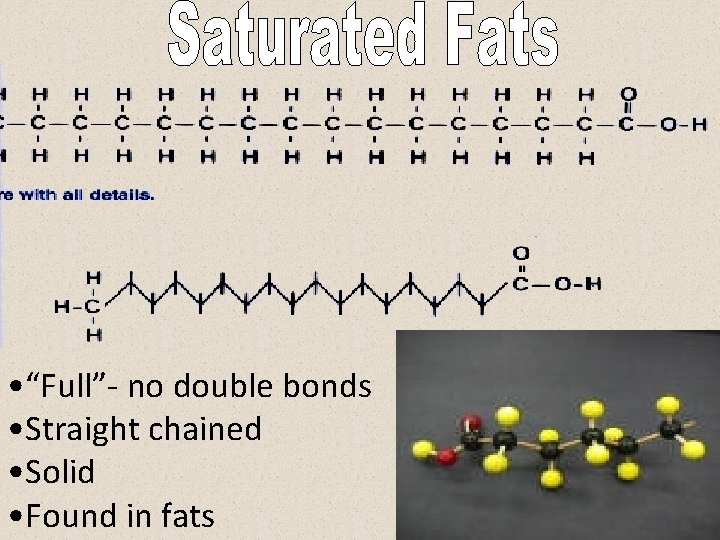
• “Full”- no double bonds • Straight chained • Solid • Found in fats
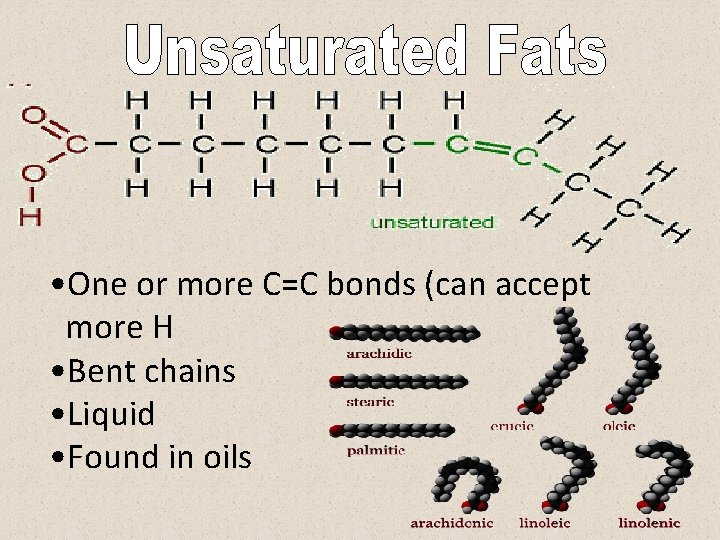
• One or more C=C bonds (can accept more H • Bent chains • Liquid • Found in oils
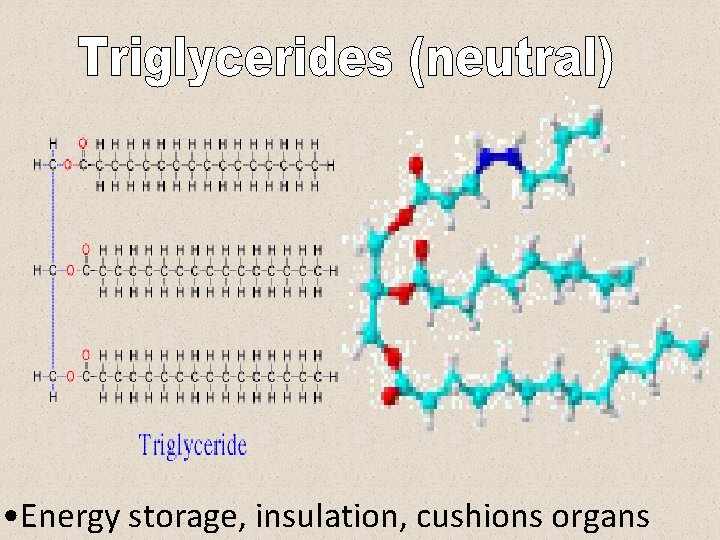
• Energy storage, insulation, cushions organs
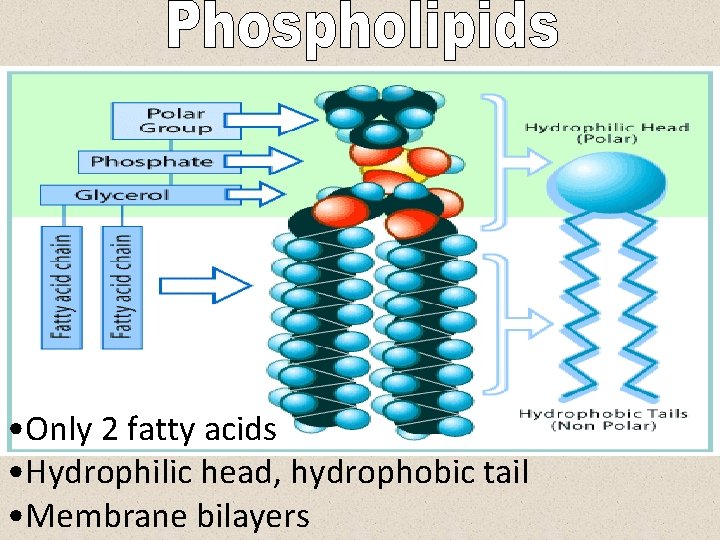
• Only 2 fatty acids • Hydrophilic head, hydrophobic tail • Membrane bilayers
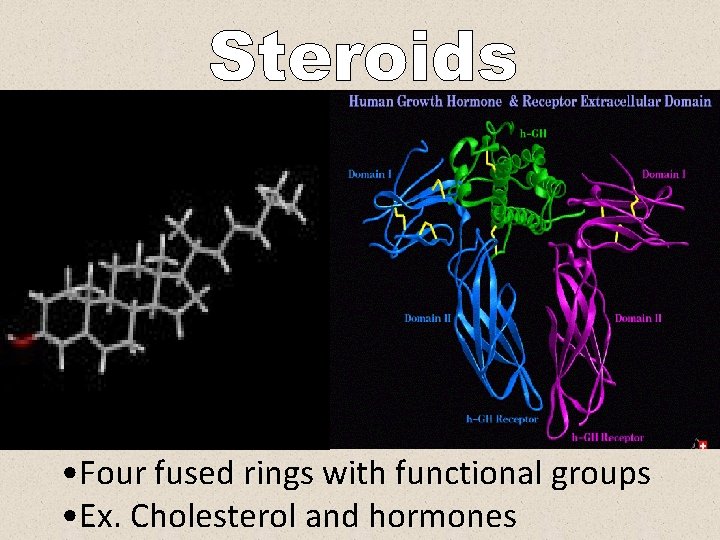
• Four fused rings with functional groups • Ex. Cholesterol and hormones
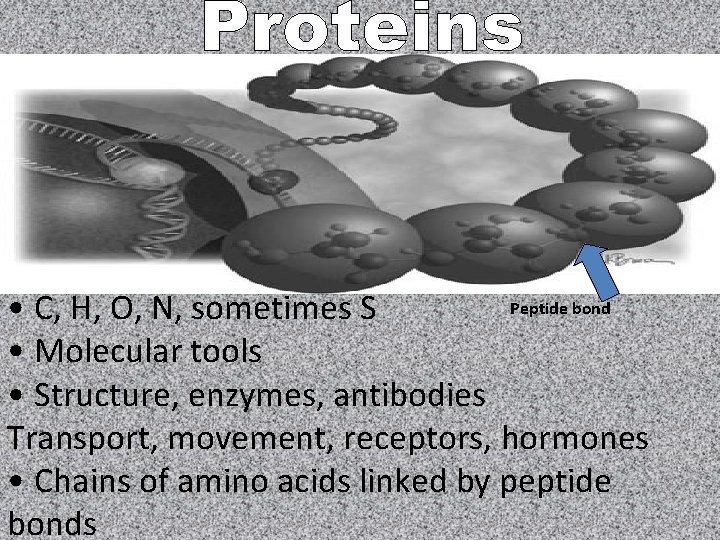
Peptide bond • C, H, O, N, sometimes S • Molecular tools • Structure, enzymes, antibodies Transport, movement, receptors, hormones • Chains of amino acids linked by peptide bonds
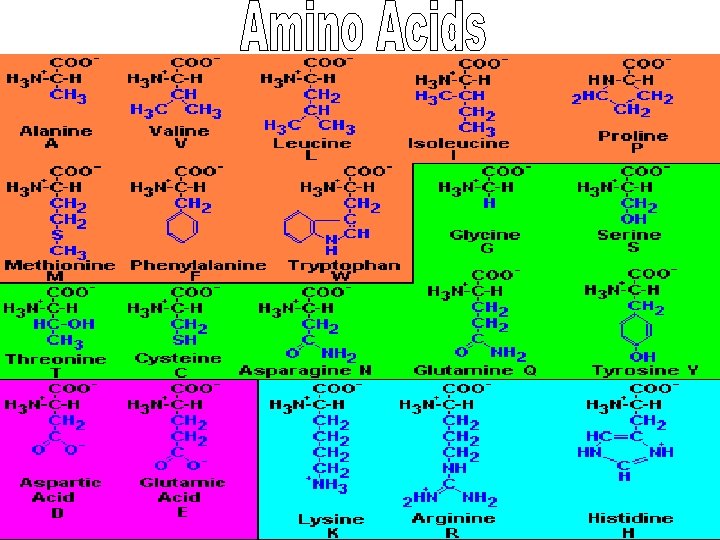
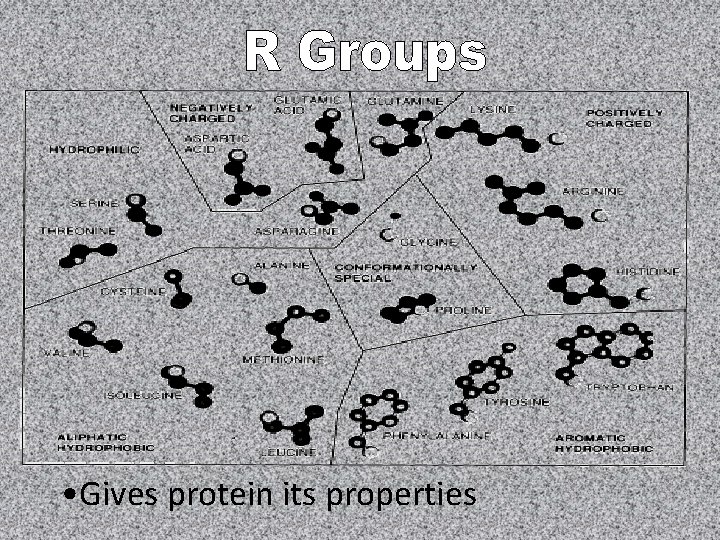
• Gives protein its properties
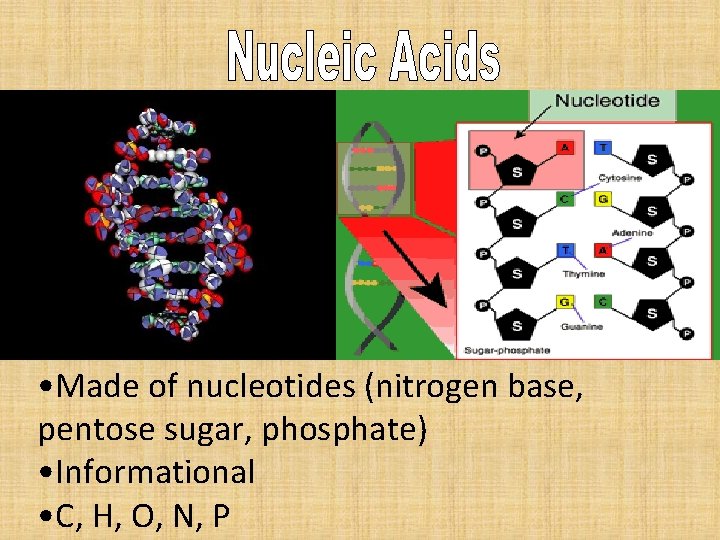
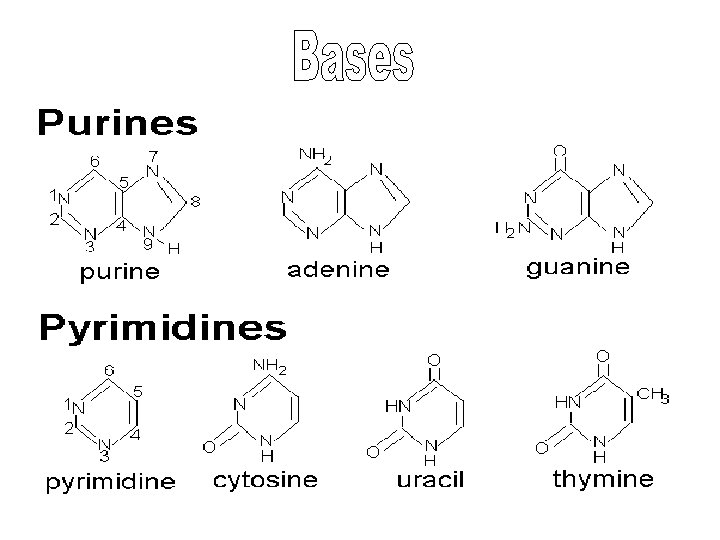
• Made of nucleotides (nitrogen base, pentose sugar, phosphate) • Informational • C, H, O, N, P
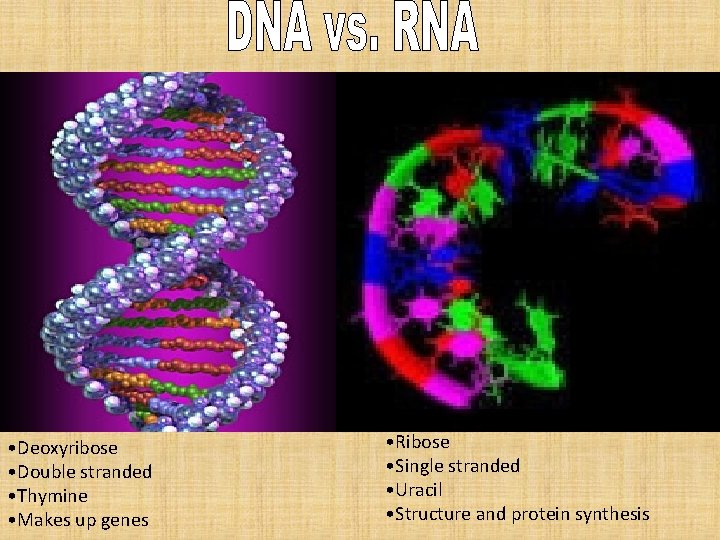
• Deoxyribose • Double stranded • Thymine • Makes up genes • Ribose • Single stranded • Uracil • Structure and protein synthesis
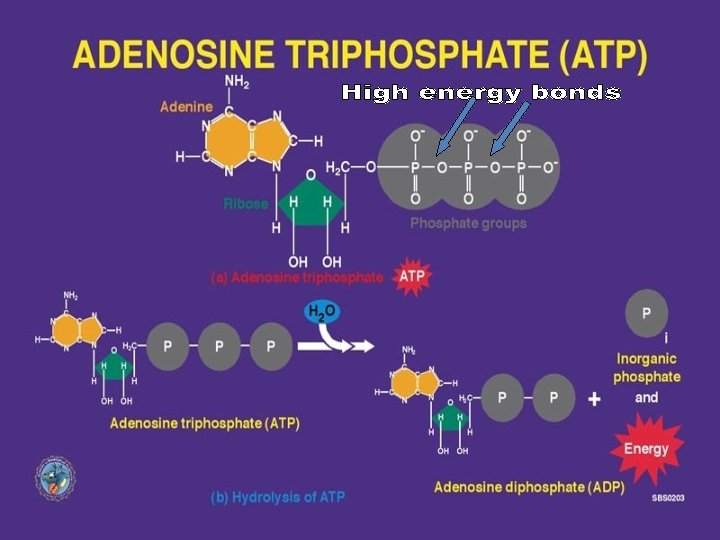
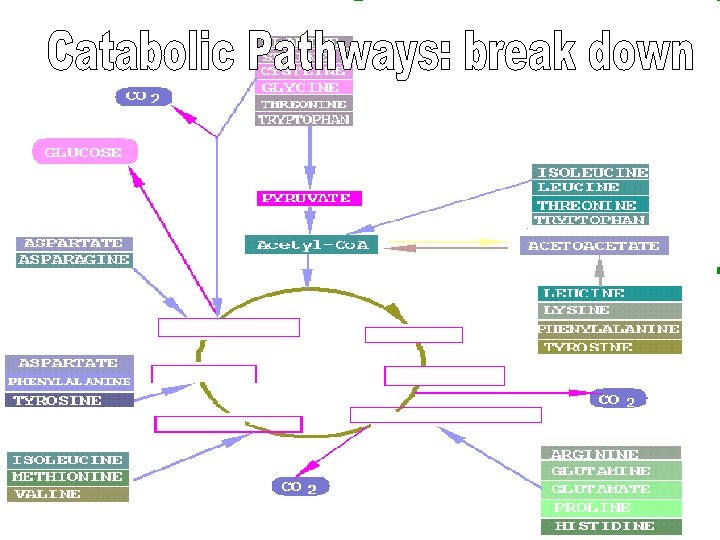
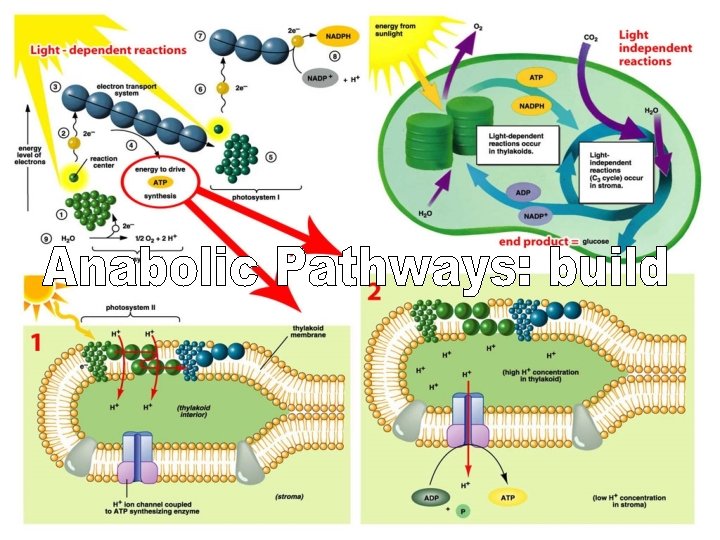


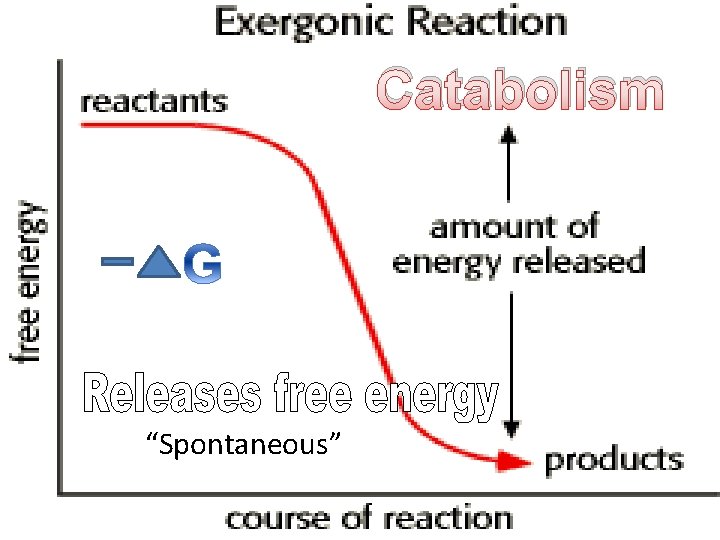
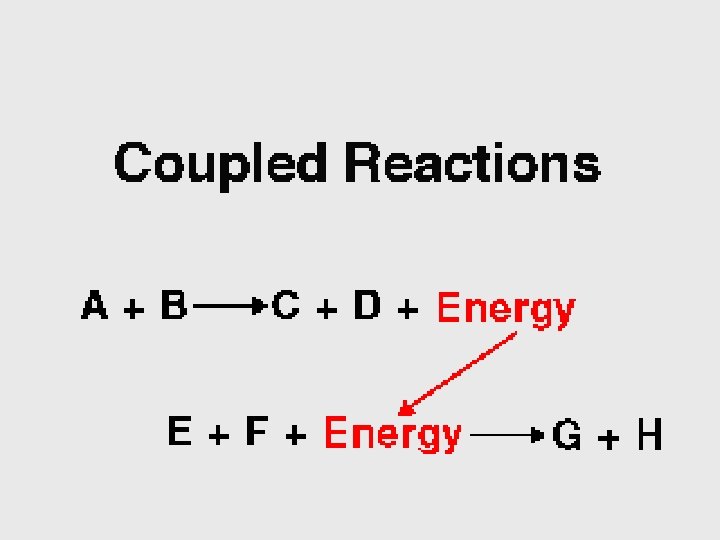
Catabolism “Spontaneous”
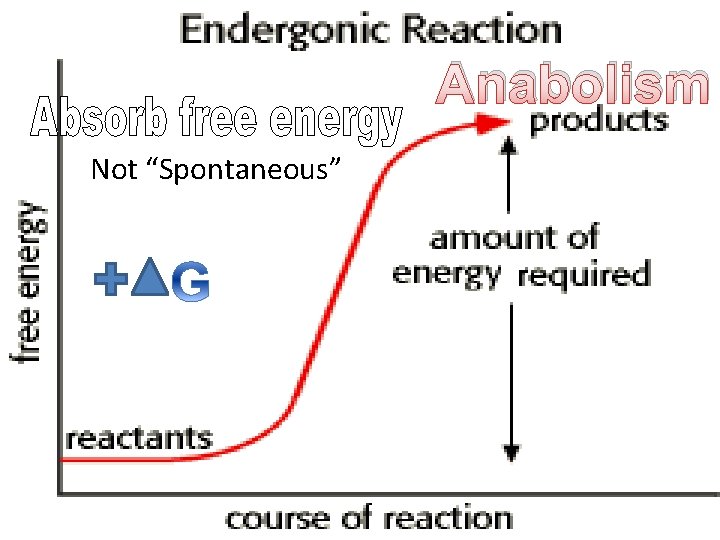
Anabolism Not “Spontaneous”
- Slides: 68