STUDY ON THE VIBRATIONAL DYNAMICS OF PHENOL AND

STUDY ON THE VIBRATIONAL DYNAMICS OF PHENOL AND PHENOL-WATER COMPLEX BY PICOSECOND TIMERESOLVED IR-UV PUMP-PROBE SPECTROSCOPY Yasunori Miyazaki, Yoshiya Inokuchi, Takayuki Ebata Department of Chemistry, Graduate school of Science, Hiroshima University
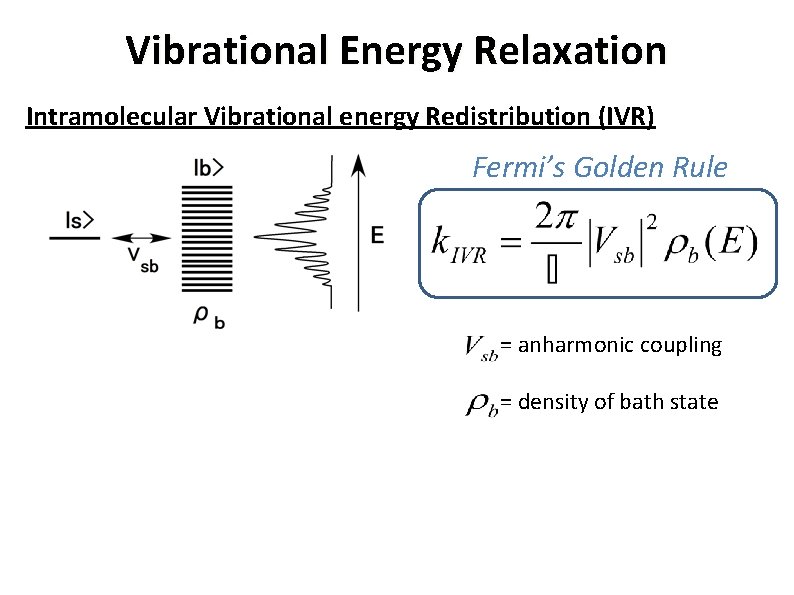
Vibrational Energy Relaxation Intramolecular Vibrational energy Redistribution (IVR) Fermi’s Golden Rule = anharmonic coupling = density of bath state

Vibrational Energy Relaxation Intramolecular Vibrational energy Redistribution (IVR) Fermi’s Golden Rule Anharmonic coupling (normal mode analysis) Anharmonic term Csij = qsqiqj = anharmonic constant Evaluation of coupling among vibrational modes: s, i, j

IR spectrum of phenol % transmittance in solution Free OH stretch Hydrogen-bonded OH stretch Large red-shift • Reduced force constant of the OH bond Spectral broadening • Vibrational Energy Relaxation • Fermi Resonance with overtone and/or combination band • Inhomogeneous broadening due to random geometries etc
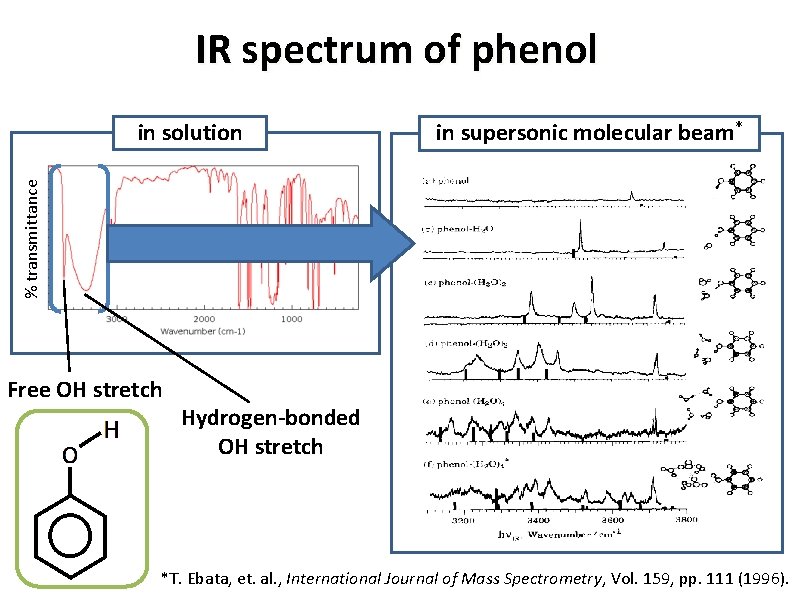
IR spectrum of phenol in supersonic molecular beam* % transmittance in solution Free OH stretch Hydrogen-bonded OH stretch *T. Ebata, et. al. , International Journal of Mass Spectrometry, Vol. 159, pp. 111 (1996).
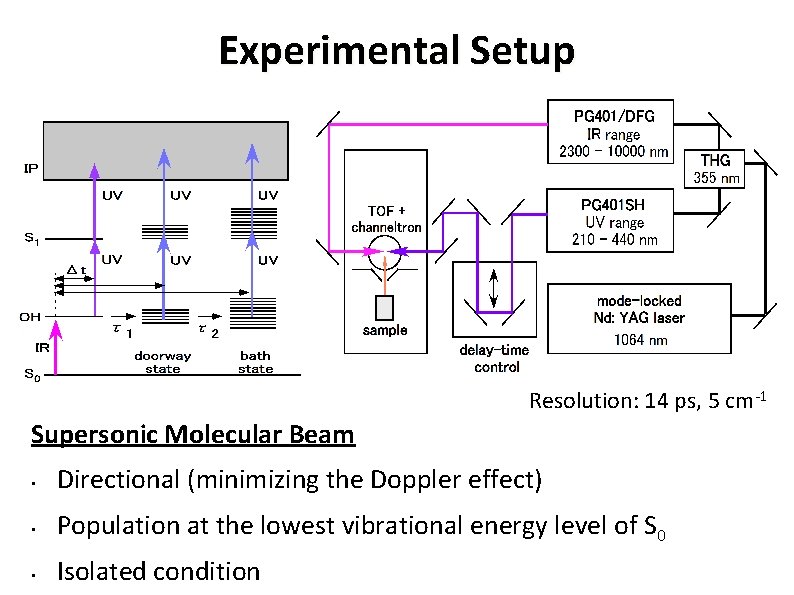
Experimental Setup Resolution: 14 ps, 5 cm-1 Supersonic Molecular Beam • Directional (minimizing the Doppler effect) • Population at the lowest vibrational energy level of S 0 • Isolated condition
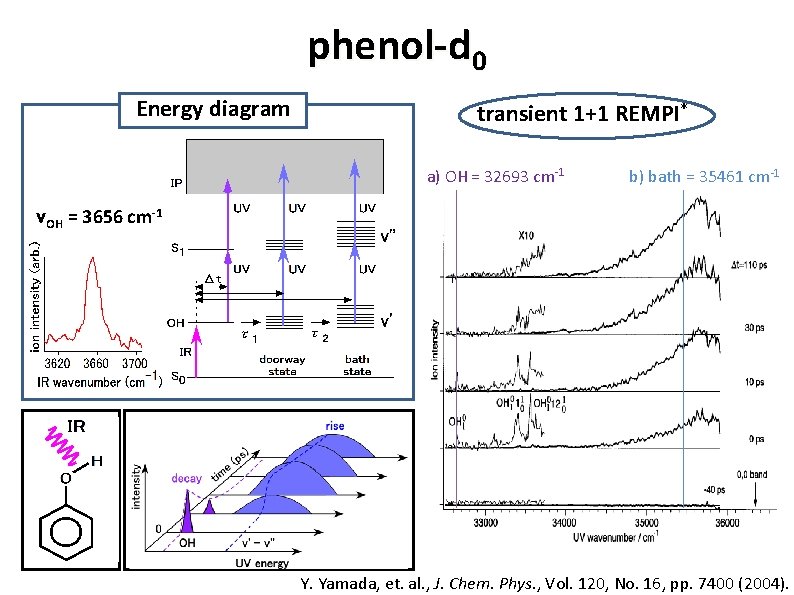
phenol-d 0 Energy diagram transient 1+1 REMPI* a) OH = 32693 cm-1 b) bath = 35461 cm-1 νOH = 3656 cm-1 Y. Yamada, et. al. , J. Chem. Phys. , Vol. 120, No. 16, pp. 7400 (2004).
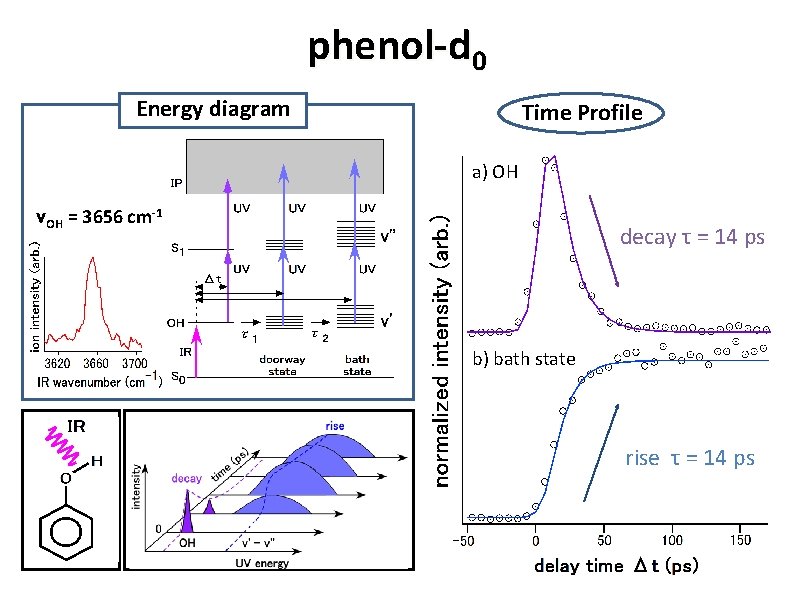
phenol-d 0 Energy diagram Time Profile a) OH νOH = 3656 cm-1 decay τ = 14 ps b) bath state rise τ = 14 ps

Summary 1 doorway state* γCH bath state* νCH *Petkovic, M. Journal of Physical Chemistry A, Vol. 116, pp. 364 -371 (2012)
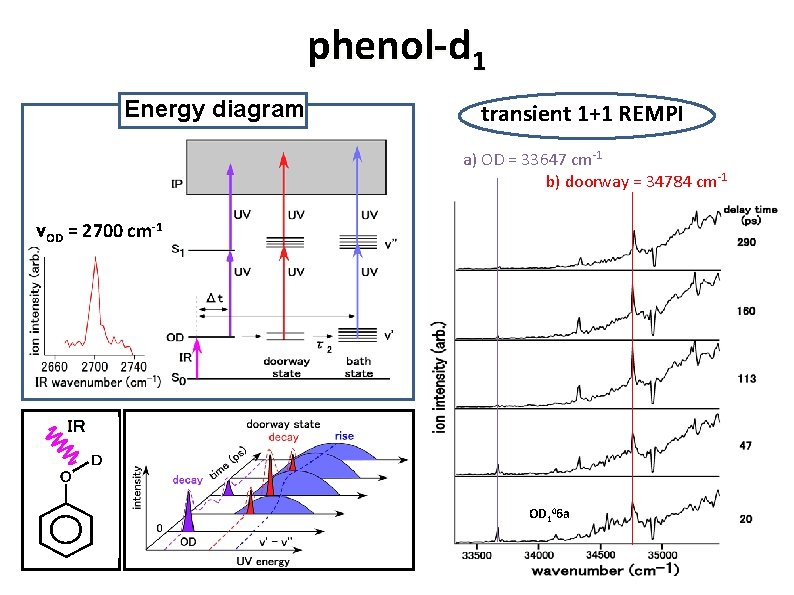
phenol-d 1 Energy diagram transient 1+1 REMPI a) OD = 33647 cm-1 b) doorway = 34784 cm-1 νOD = 2700 cm-1 OD 106 a
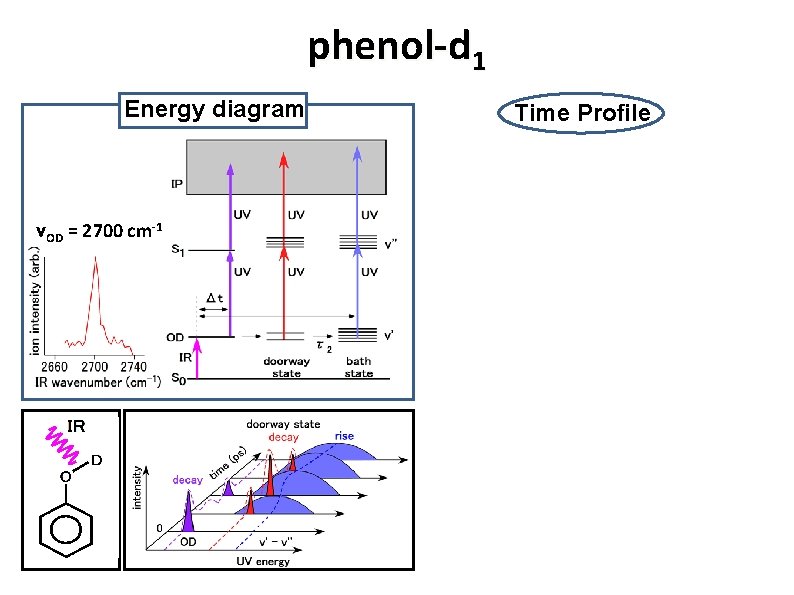
phenol-d 1 Energy diagram νOD = 2700 cm-1 IR O D Time Profile
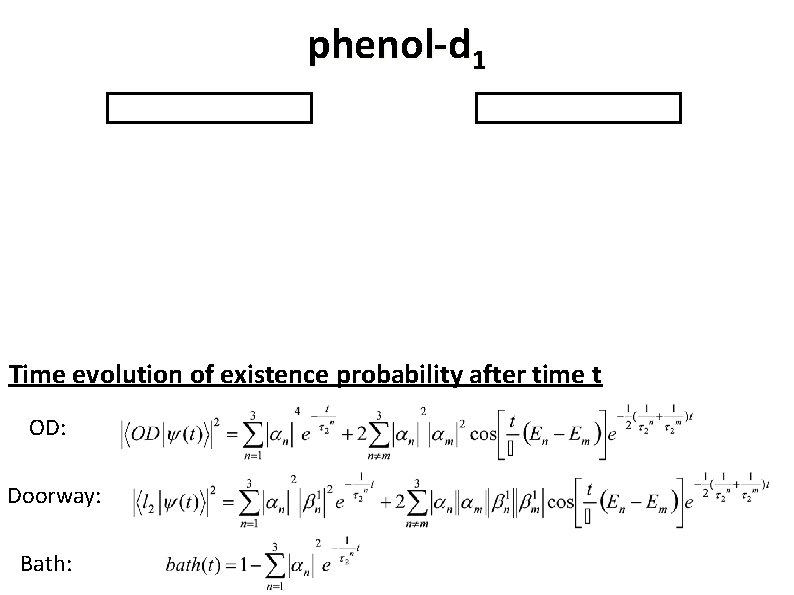
phenol-d 1 Time evolution of existence probability after time t OD: Doorway: Bath:
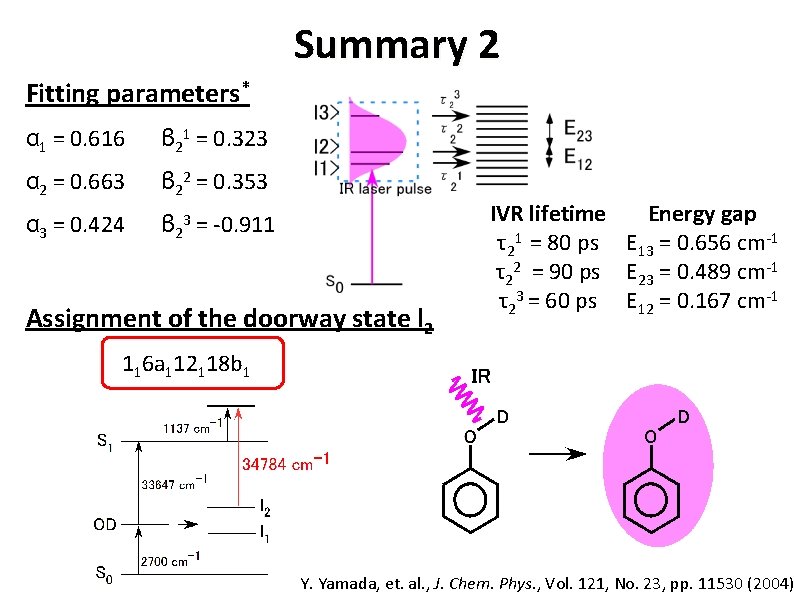
Summary 2 Fitting parameters* α 1 = 0. 616 β 21 = 0. 323 α 2 = 0. 663 β 22 = 0. 353 α 3 = 0. 424 β 23 = -0. 911 Assignment of the doorway state l 2 IVR lifetime Energy gap τ21 = 80 ps E 13 = 0. 656 cm-1 τ22 = 90 ps E 23 = 0. 489 cm-1 τ23 = 60 ps E 12 = 0. 167 cm-1 116 a 112118 b 1 Y. Yamada, et. al. , J. Chem. Phys. , Vol. 121, No. 23, pp. 11530 (2004)
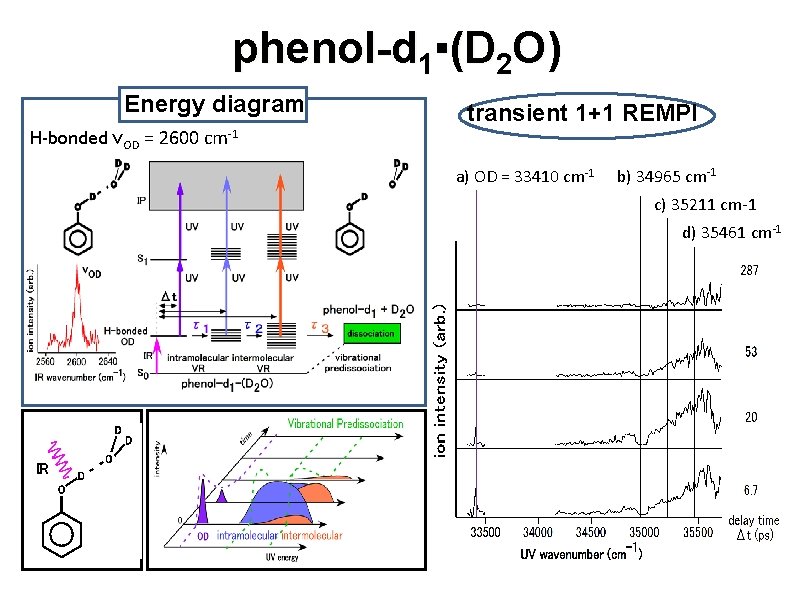
phenol-d 1▪(D 2 O) Energy diagram H-bonded νOD = 2600 cm-1 transient 1+1 REMPI a) OD = 33410 cm-1 b) 34965 cm-1 c) 35211 cm-1 d) 35461 cm-1 O D IR O D D
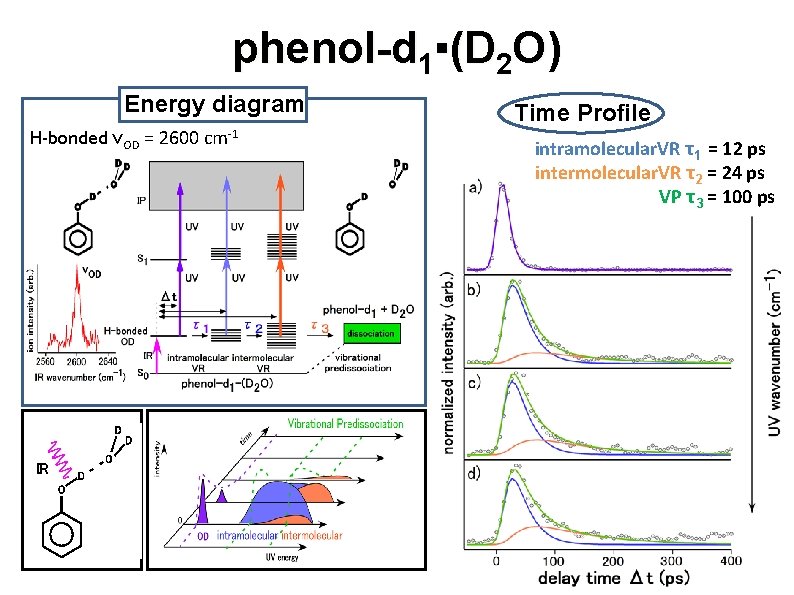
phenol-d 1▪(D 2 O) Energy diagram H-bonded νOD = 2600 cm-1 O D IR O D D Time Profile intramolecular. VR τ1 = 12 ps intermolecular. VR τ2 = 24 ps VP τ3 = 100 ps
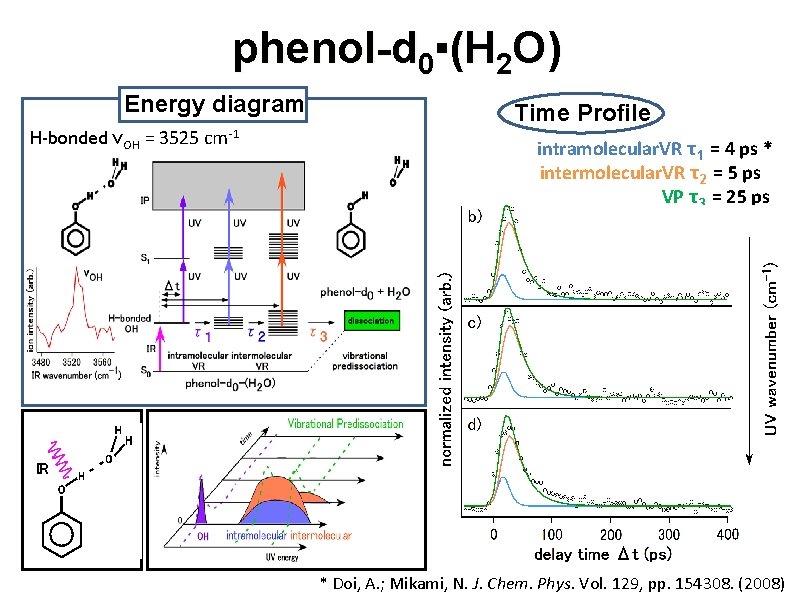
phenol-d 0▪(H 2 O) Energy diagram H-bonded νOH = 3525 cm-1 Time Profile intramolecular. VR τ1 = 4 ps * intermolecular. VR τ2 = 5 ps VP τ3 = 25 ps O D IR O D D * Doi, A. ; Mikami, N. J. Chem. Phys. Vol. 129, pp. 154308. (2008)
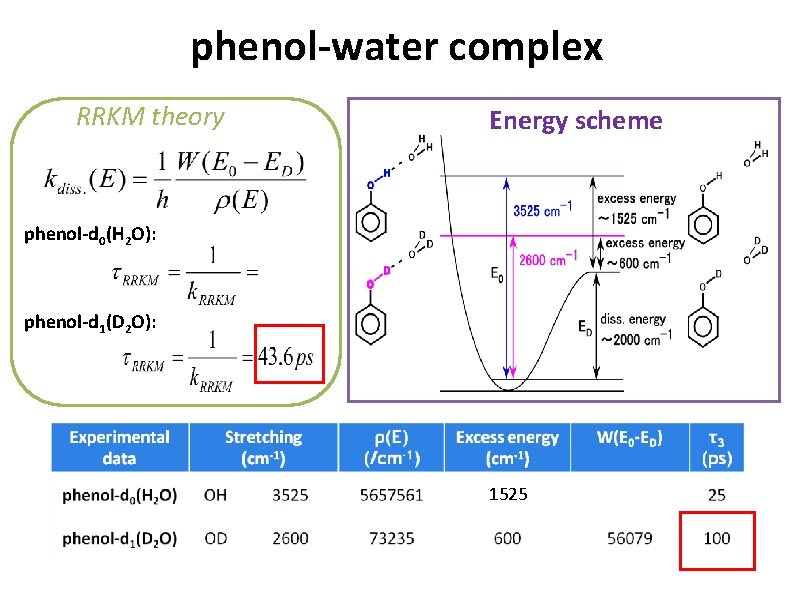
phenol-water complex RRKM theory Energy scheme phenol-d 0(H 2 O): phenol-d 1(D 2 O): 1525
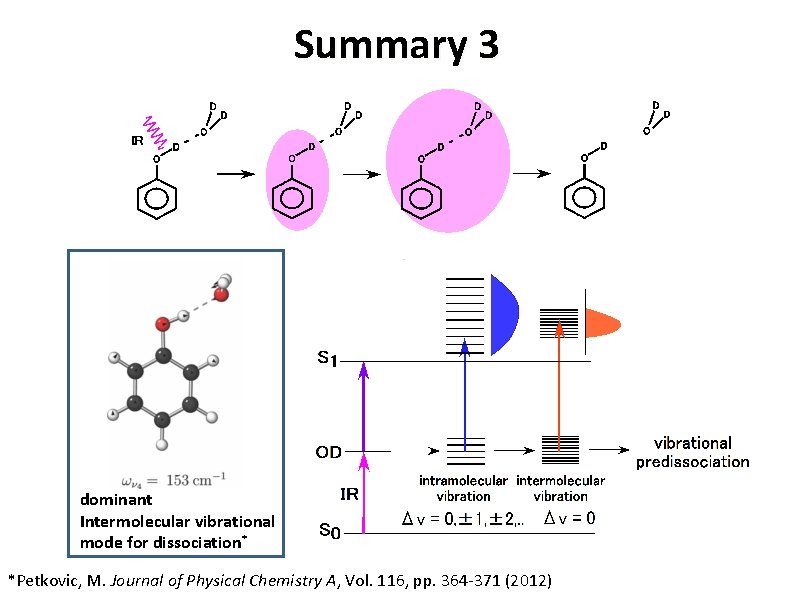
Summary 3 dominant Intermolecular vibrational mode for dissociation* *Petkovic, M. Journal of Physical Chemistry A, Vol. 116, pp. 364 -371 (2012)
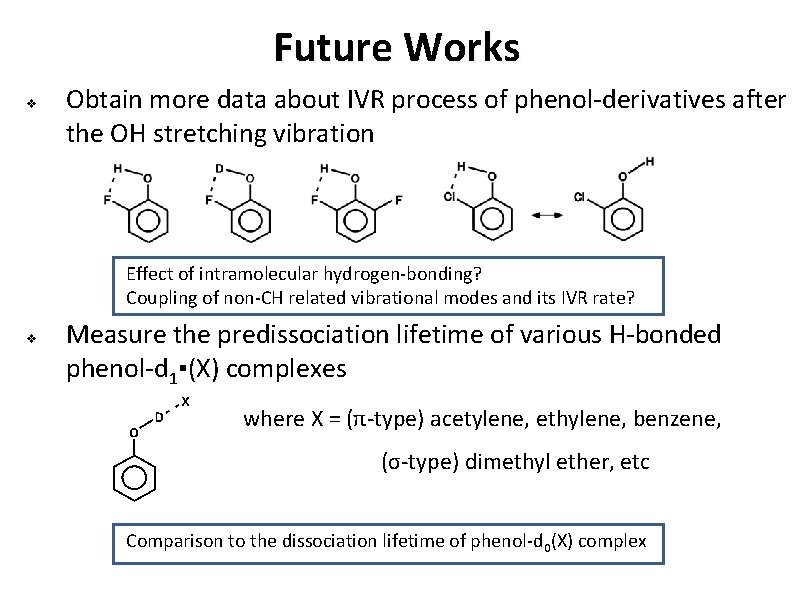
Future Works v Obtain more data about IVR process of phenol-derivatives after the OH stretching vibration Effect of intramolecular hydrogen-bonding? Coupling of non-CH related vibrational modes and its IVR rate? v Measure the predissociation lifetime of various H-bonded phenol-d 1▪(X) complexes where X = (π-type) acetylene, ethylene, benzene, (σ-type) dimethyl ether, etc Comparison to the dissociation lifetime of phenol-d 0(X) complex
- Slides: 19