Study of Cells Tissues Objectives of CellTissue Studies

Study of Cells & Tissues

Objectives of Cell/Tissue Studies • To Gain insight into detailed morphological Features. • Morphology is a rough indicator of Function. • To Compare Normal Structures Vs Abnormal (Pathology). • To Understand Functional aspect.

Microscopy • Trans-illumination of thin sections that have been preserved as close as possible to Original Form In The Living. • Stains are used to show contrast colors. • Resolution: The smallest distance between two structures at which they can be seen as two separate objects. • In Light Microscopy: 0. 2µm Electron: 3 nm. • Magnification: Light 1000 -1500. • Electron; 120, 000.

The Light Microscope Can Resolve Details 0. 2 µ Apart • In general, a given type of radiation cannot be used to probe structural details much smaller than its own wavelength. This is a fundamental limitation of all microscopes. The ultimate limit to the Resolution of a light microscope is therefore set by the Wavelength Of Visible Light, which ranges from about 0. 4 µ m (for violet) to 0. 7 µ m (for deep red). In practical terms, bacteria and mitochondria, which are about 500 nm (0. 5 µ m) wide, are generally the smallest objects whose shape can be clearly discerned in the light microscope
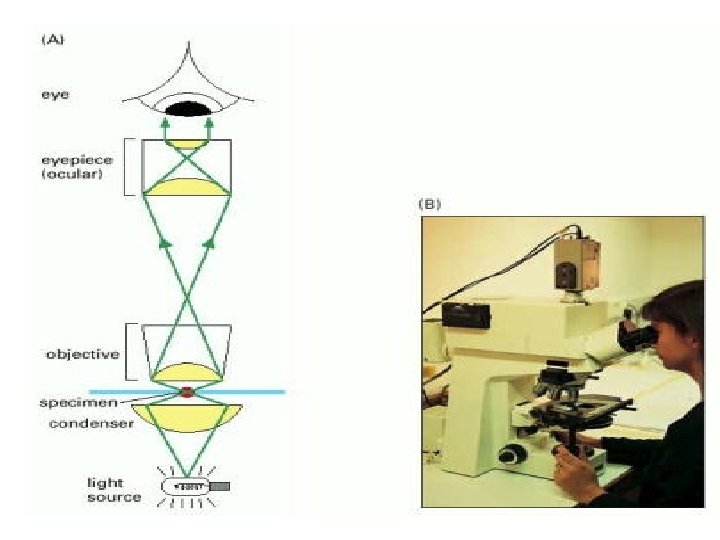

Microscopy • Light Microscopy • Use of Light Beam • Magnification =10001500 times. • Resolution =0. 2µm • Section Thickness=1 -90 µm. • Fixatives: 4% Formaldehyde • Embedding: Parafin • Electron Microscopy • Use of Electron Beam • Magnification =120000 times. • Resolution =2 nm • Section Thickness=2 -10 nm. • Fixatives: 4% Glutaraldehyde. • Embedding: Resins

Steps in Tissue Preparation for Microscopic Study • 1. Collection of Tissue: Soon after Death. • 2. Cutting: Thickness should not exceed. 5 cm (Light) & 1 -2 -mm (Electron). Fixative may not penetrate whole tissue. • 3. Fixation: Chemicals that interact with amine groups OR Cross-link with Proteins. NOTE No fixatives for Enzymatic Studies & During Surgical Procedures We use Freeze Fracture ( Immerse tissue in Liquid Nitrogen -70 -800 • 4. Dehydration: Removal of water by immersing tissue block in gradual concentration of Alcohol (70%100%). • 5. Clearing: Removal of Alcohol with Xylene. Because Alcohol do not mix with Paraffin & To give the specimen transparent look

Steps in Tissue Preparation for Microscopic Study • 6. Embedding: Paraffin/Resin. Maintain internal architect of the tissue and provide the block with certain solidity to facilitate its sectioning • 7. Sectioning: Use of Microtome • 8. Staining: Acidophils/Basophils stains OR Mixed types as Hematoxylin Eosin. • 9. Mounting on Glass Slides.

Stains Natural Or Chemical Dyes • Classified as Basophilic OR Eosinophilic • Hematoxylin (Basic Dye stains acidophilic structures) & Eosin ( Acidic Dye stain Basic structures as Cytoplasmic proteins Pink Colour (H&E). • Periodic Acid-Schiff Reaction (PAS): Selectively stains carbohydrates (Mucin of Goblet Cells) as Deep Red Color called Magenta. Also Basement membranes, Brush border cells of GIT, Kidneys, Glycogen & Cartilages are PAS positive. • Masson’s Trichrome: Used to stain support tissue; Gives three colors; Nuclei blue, Collagen green, and Cytoplasm muscle, RBS, Keratin Bright Red color. • Van Gieson: CT stain. Collagen Red, Nuclei Blue. , Cytoplasm & RBCs yellow. • Azan: CT stain. Special Epithelium stain. Nuclei Bright red, BM & Mucin Blue. RBCs Orange. • Giemsa stain: Standard Blood Cells Smear & Bone Marrow. . Nuclei Blue. Cytoplasm Pale Blue. RBCs pink. • Sliver & Glod: Used specially for Nervous tissue studyies • Nissl Stain Used to demonstrate r. ER of neurons. .
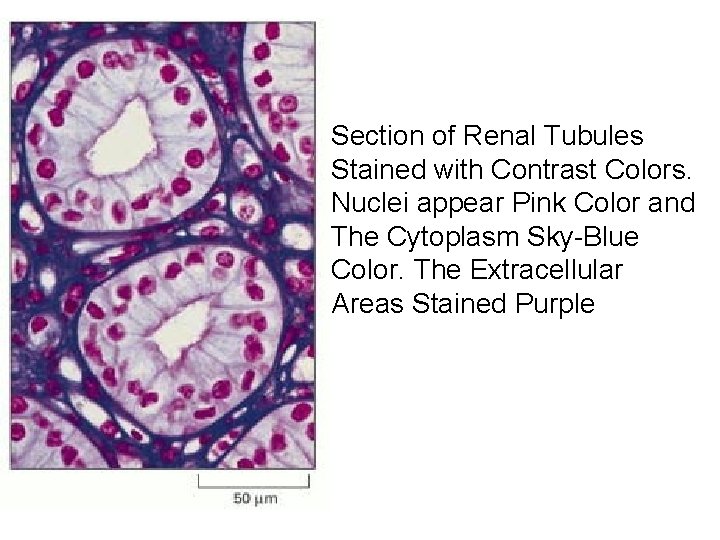
Section of Renal Tubules Stained with Contrast Colors. Nuclei appear Pink Color and The Cytoplasm Sky-Blue Color. The Extracellular Areas Stained Purple
Types of Light Microscopy 1. 2. 3. 4. 5. Ordinary: Light beam transmitted through tissue. Phase Contrast: Lens can show different densities of light that passed through different structures of cells (Living cell Study) Polarizing Microscopy: Light passes through different parts of cell. Then passes between two filters which are perpendicular to each other. Different structures appear as light against dark back ground (Collagen). Confocal: Thin beam of light illuminate tissue. Then passes through very thin pinhole, So only illuminated part is in focus. Provides serial sections of specimen and 3 -dimensional image could be constructed. Fluorescent: fluorescent dye molecules are excited. Decay of the molecule from the excited state to an intermediate excited state produces a photon of a wavelength longer than that of the excitation source, which can easily be detected and separated
Phase Contrast Microscopy • When light passes through a living cell, the phase of the light wave is changed according to the cell's Refractive Index: light passing through a relatively thick or dense part of the cell, such as the nucleus, is retarded; its phase, consequently, is shifted relative to light that has passed through an adjacent thinner region of the cytoplasm

Dark-field microscope The illuminating rays of light are directed from the side so that only scattered light enters the microscope lenses. Consequently, the cell appears as a Bright Object Against A Dark Background.
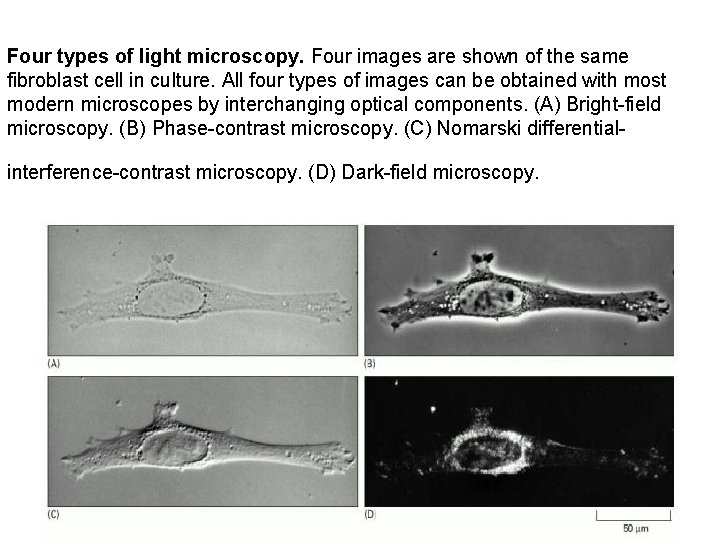
Four types of light microscopy. Four images are shown of the same fibroblast cell in culture. All four types of images can be obtained with most modern microscopes by interchanging optical components. (A) Bright-field microscopy. (B) Phase-contrast microscopy. (C) Nomarski differentialinterference-contrast microscopy. (D) Dark-field microscopy.
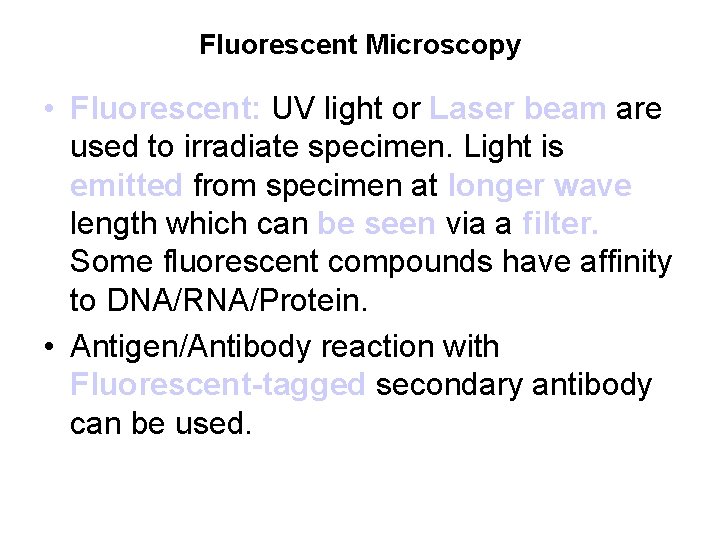
Fluorescent Microscopy • Fluorescent: UV light or Laser beam are used to irradiate specimen. Light is emitted from specimen at longer wave length which can be seen via a filter. Some fluorescent compounds have affinity to DNA/RNA/Protein. • Antigen/Antibody reaction with Fluorescent-tagged secondary antibody can be used.
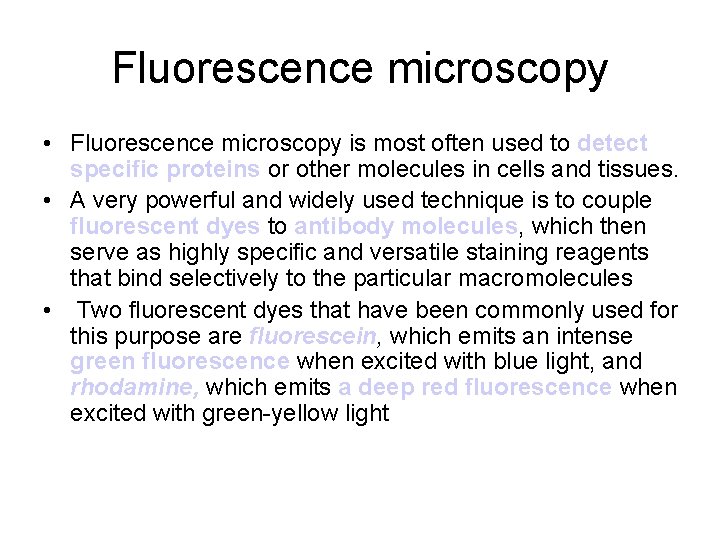
Fluorescence microscopy • Fluorescence microscopy is most often used to detect specific proteins or other molecules in cells and tissues. • A very powerful and widely used technique is to couple fluorescent dyes to antibody molecules, which then serve as highly specific and versatile staining reagents that bind selectively to the particular macromolecules • Two fluorescent dyes that have been commonly used for this purpose are fluorescein, which emits an intense green fluorescence when excited with blue light, and rhodamine, which emits a deep red fluorescence when excited with green-yellow light
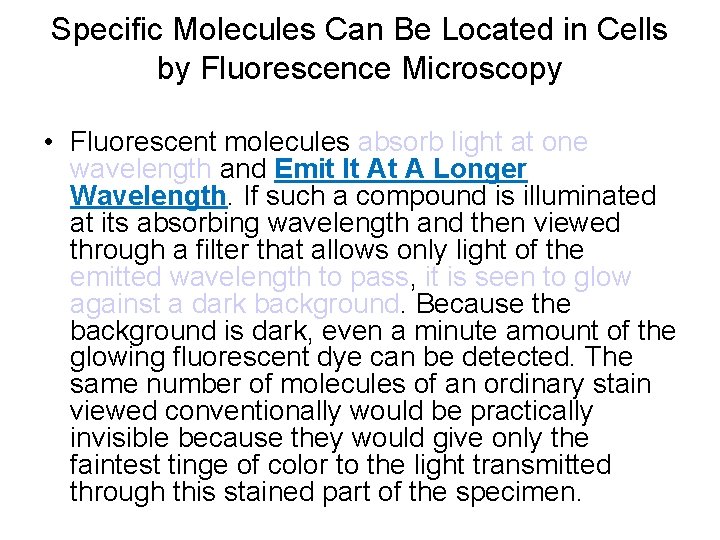
Specific Molecules Can Be Located in Cells by Fluorescence Microscopy • Fluorescent molecules absorb light at one wavelength and Emit It At A Longer Wavelength. If such a compound is illuminated at its absorbing wavelength and then viewed through a filter that allows only light of the emitted wavelength to pass, it is seen to glow against a dark background. Because the background is dark, even a minute amount of the glowing fluorescent dye can be detected. The same number of molecules of an ordinary stain viewed conventionally would be practically invisible because they would give only the faintest tinge of color to the light transmitted through this stained part of the specimen.
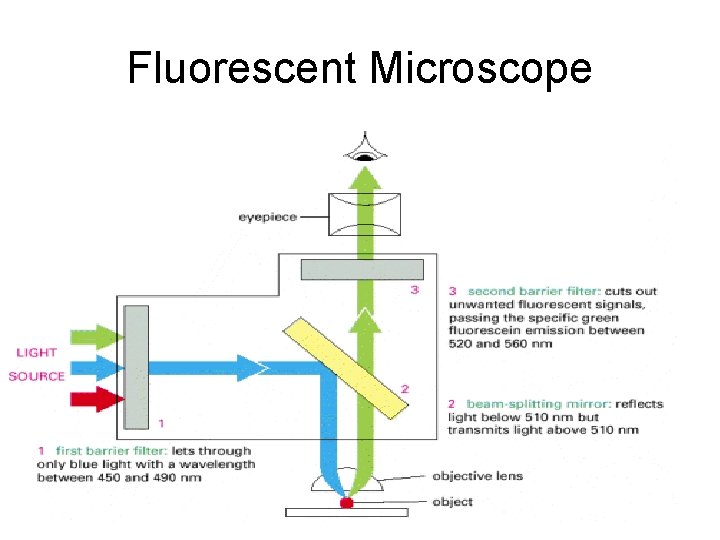
Fluorescent Microscope
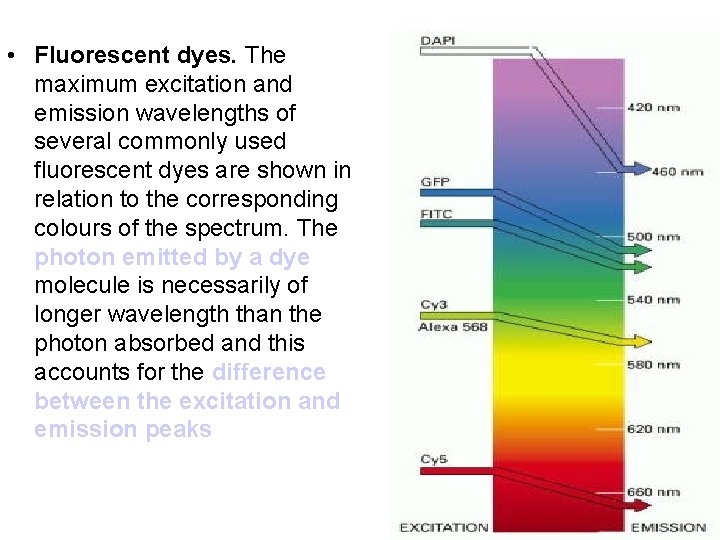
• Fluorescent dyes. The maximum excitation and emission wavelengths of several commonly used fluorescent dyes are shown in relation to the corresponding colours of the spectrum. The photon emitted by a dye molecule is necessarily of longer wavelength than the photon absorbed and this accounts for the difference between the excitation and emission peaks
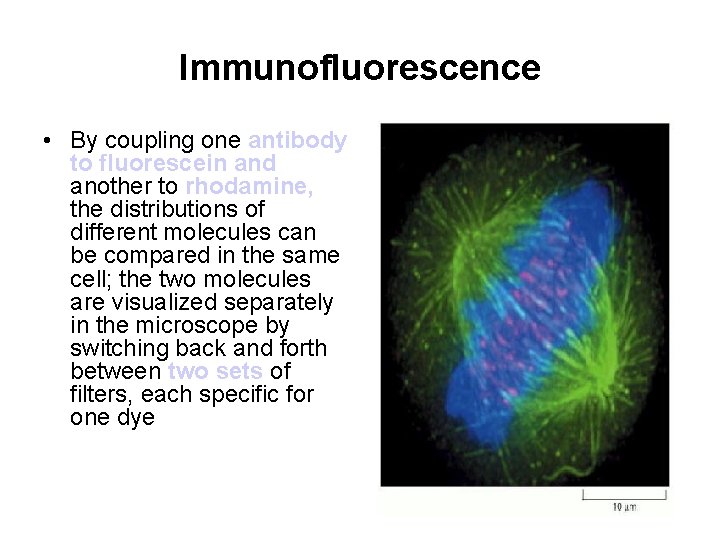
Immunofluorescence • By coupling one antibody to fluorescein and another to rhodamine, the distributions of different molecules can be compared in the same cell; the two molecules are visualized separately in the microscope by switching back and forth between two sets of filters, each specific for one dye

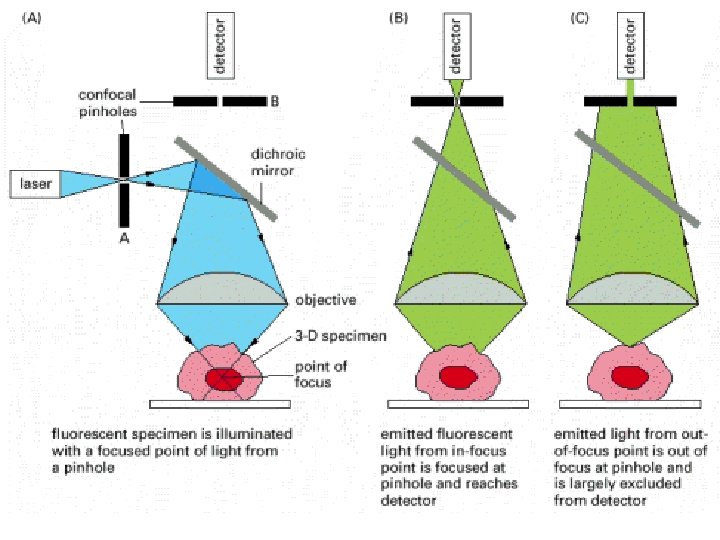
Three-dimensional Confocal microscopic imaging • For ordinary light microscopy, a tissue has to be sliced into thin sections to be examined. In the process of sectioning, information about the third dimension is lost. How, then, can one get a picture of the three-dimensional of a cell or tissue, with out sectioning? Although an optical microscope is focused on a particular focal plane within complex three-dimensional specimens, all the light originating from these regions contributes to the image as "out-of -focus" blur. • Optical Microscopy focus on a chosen plane in a thick specimen while rejecting the light that comes from out-of-focus regions above and below that plane.
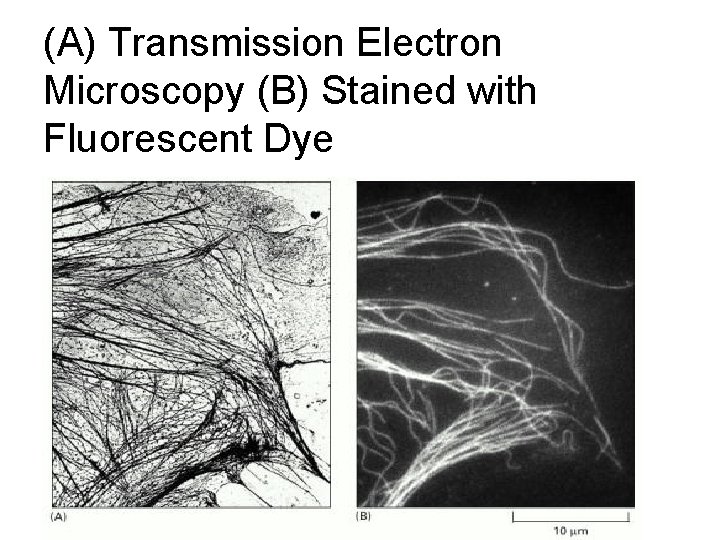
(A) Transmission Electron Microscopy (B) Stained with Fluorescent Dye
Cell Culture • Objectives: • To obtain as much information as possible about an individual cell type • The cells can be watched continuously under the microscope or analyzed biochemically, and the effects of adding or removing specific molecules, such as hormones or growth factors, can be explored. • In addition, by mixing two cell types, the interactions between one cell type and another can be studied. Experiments performed on cultured cells are sometimes said to be carried out in vitro (literally, "in glass") to contrast them with experiments using intact organisms, which are said to be carried out in vivo (literally, "in the living organism").

Cell Culture • Primary cultures: Cultures prepared directly from the tissues of an organism, that is, without cell proliferation. In most cases, cells in primary cultures can be removed from the culture dish and made to proliferate to form a large number of so-called secondary cultures. • Such cells often display many of the differentiated properties appropriate to their origin: eg: fibroblasts continue to secrete collagen; cells derived from embryonic skeletal muscle fuse to form muscle fibers that contract spontaneously in the culture dish; nerve cells extend axons that are electrically excitable and make synapses with other nerve cells; and epithelial cells form extensive sheets with many of the properties of an intact epithelium
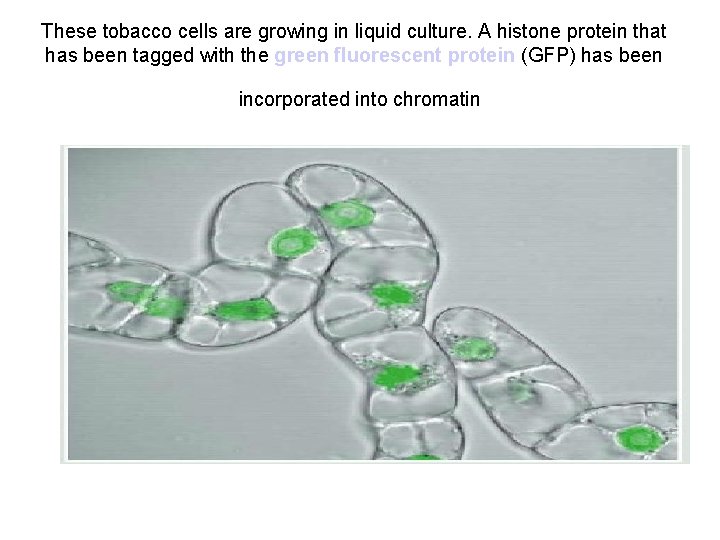
These tobacco cells are growing in liquid culture. A histone protein that has been tagged with the green fluorescent protein (GFP) has been incorporated into chromatin
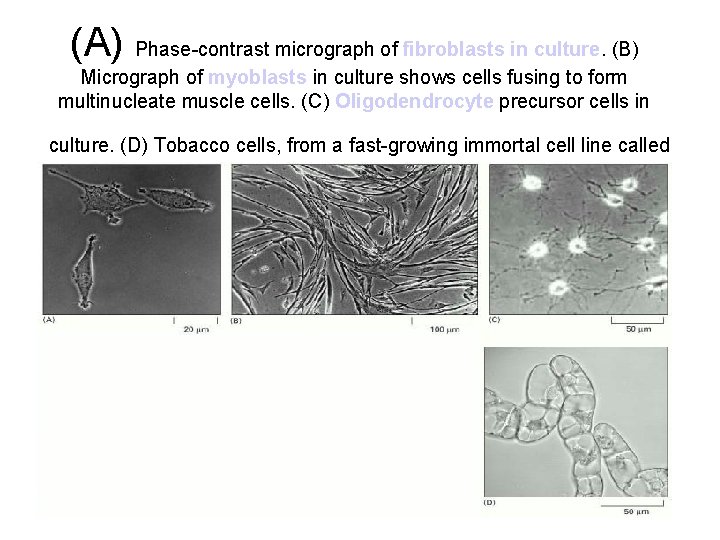
(A) Phase-contrast micrograph of fibroblasts in culture. (B) Micrograph of myoblasts in culture shows cells fusing to form multinucleate muscle cells. (C) Oligodendrocyte precursor cells in culture. (D) Tobacco cells, from a fast-growing immortal cell line called
Fractionation of Cells • Cells can be broken up in various ways: they can be subjected to osmotic shock or ultrasonic vibration, or ground up in a blender. • These procedures break many of the membranes of the cell (including the plasma membrane and membranes of the endoplasmic reticulum) into fragments that immediately reseal to form small closed vesicles. • That procedures leave organelles such as nuclei, mitochondria, the Golgi apparatus, lysosomes, and peroxisomes largely intact. • The suspension of cells is thereby reduced to a thick slurry (called a homogenate or extract) that contains a variety of membrane-enclosed organelles, each with a distinctive size, charge, and density. • The various components including the vesicles derived from the endoplasmic reticulum, called microsomes retain most of their original biochemical properties.
Cell Fractionation • • Homogenization. Centrifugation. At 1000 g Nuclei Precipitate. At 10000 g; Mitochondria & Lysosomes Precipitate. • At 100000 g : Microsomes -ER & Ribosomes Precipitate.
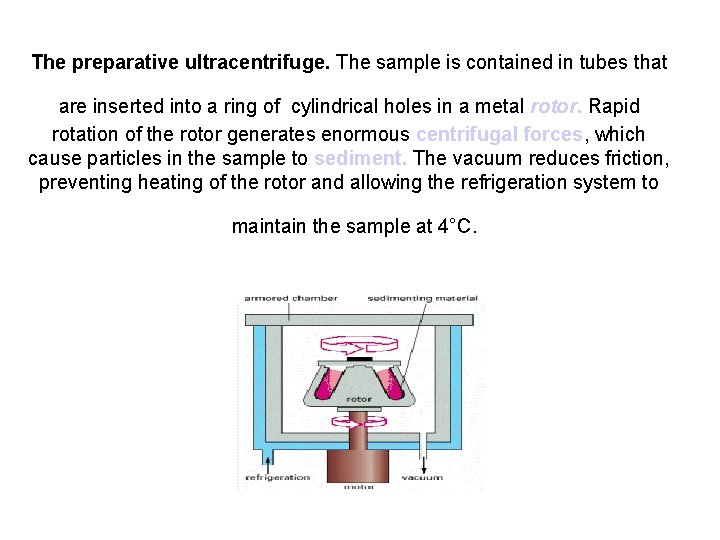
The preparative ultracentrifuge. The sample is contained in tubes that are inserted into a ring of cylindrical holes in a metal rotor. Rapid rotation of the rotor generates enormous centrifugal forces, which cause particles in the sample to sediment. The vacuum reduces friction, preventing heating of the rotor and allowing the refrigeration system to maintain the sample at 4°C.
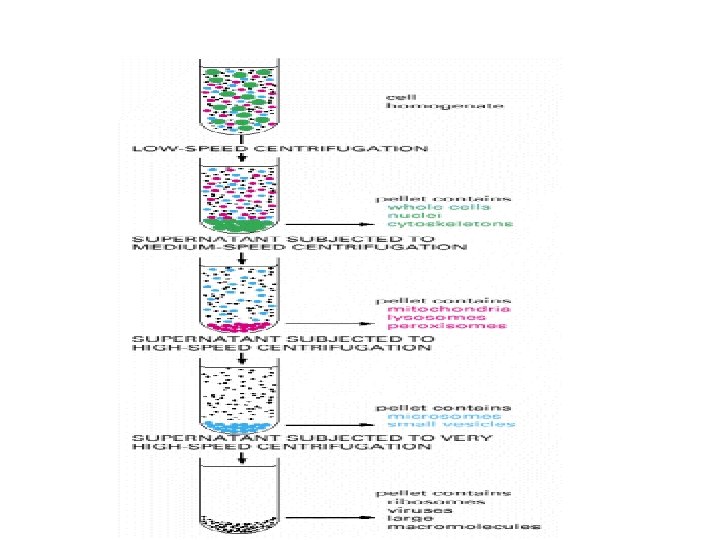
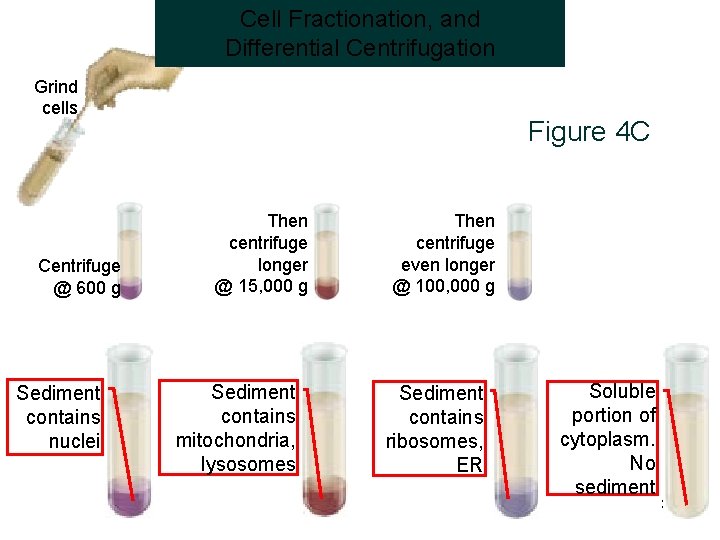
Cell Fractionation, and Differential Centrifugation Grind cells Centrifuge @ 600 g Sediment contains nuclei Figure 4 C Then centrifuge longer @ 15, 000 g Sediment contains mitochondria, lysosomes Then centrifuge even longer @ 100, 000 g Sediment contains ribosomes, ER Soluble portion of cytoplasm. No sediment 32

Antibodies Can Be Used to Detect Specific Molecules • Antibodies are proteins produced by the vertebrate immune system as a defense against infection). Each with a different binding site that recognizes a specific target molecule (or antigen). The precise antigen specificity of antibodies makes them powerful tools for the cell biologist. When labeled with fluorescent dyes, they are invaluable for locating specific molecules in cells by fluorescence microscopy
Antibodies • Antibodies are made most simply by injecting a sample of the antigen several times into an animal such as a rabbit or a goat and then collecting the antibody-rich serum. This antiserum contains a heterogeneous mixture of antibodies, each produced by a different antibody-secreting cell (a B lymphocyte). The different antibodies recognize various parts of the antigen molecule (called an antigenic determinant, or epitope), as well as impurities in the antigen preparation. • The specificity of an antiserum for a particular antigen can sometimes be sharpened by removing the unwanted antibody molecules that bind to other molecules.
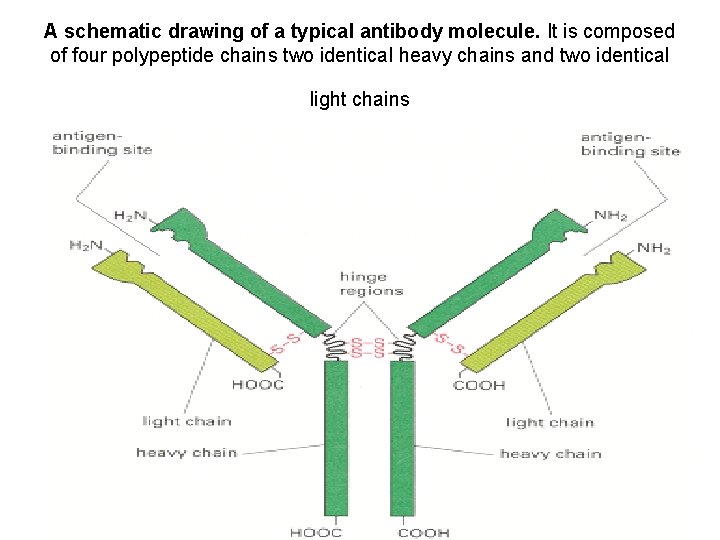
A schematic drawing of a typical antibody molecule. It is composed of four polypeptide chains two identical heavy chains and two identical light chains

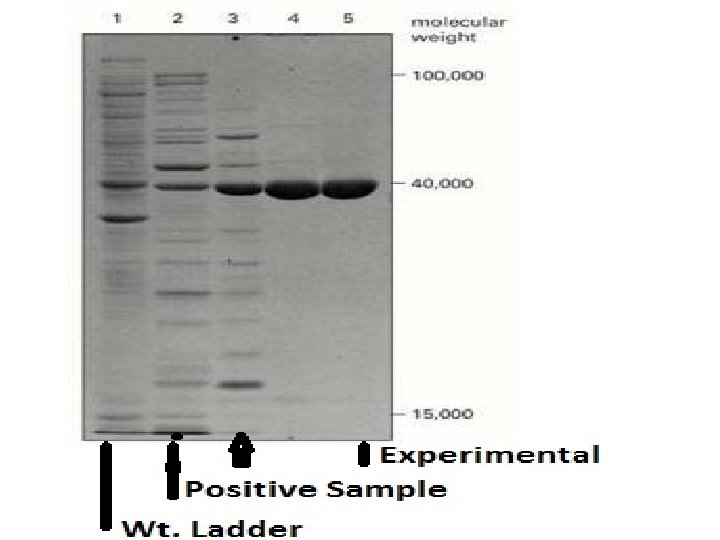
Steps in Study of Protein Expression • 1. • 2. • 3. • • • Collect Samples (Control Vs. Experimental) Homogenize Tissue, Extract Protein Then Denature. Inject Equal Amounts Of Protein In Different Polyacramide Gel. 4. Electrophorosis: Use Electric Field. Proteins Migrate According To Molecular Weight. 5. We Can Add; A Molecular Ladder, Positive Sample. 6. Transfer The Protein Bands To Nitrocellulose Membrane 7. Add Primary Antibody Then Secondary Antibody With A Tag To Visualize The Protein Band. Compare The Different Protein bands & Draw Conclusions
Electrophoresis • Proteins usually possess a net positive or negative charge, depending on the mixture of charged amino acids they contain. • When an electric field is applied to a solution containing a protein molecule, the protein migrates at a rate that depends on its net charge and on its Molecular Weight. • It uses a highly cross-linked gel of polyacrylamide as the inert matrix through which the proteins migrate.
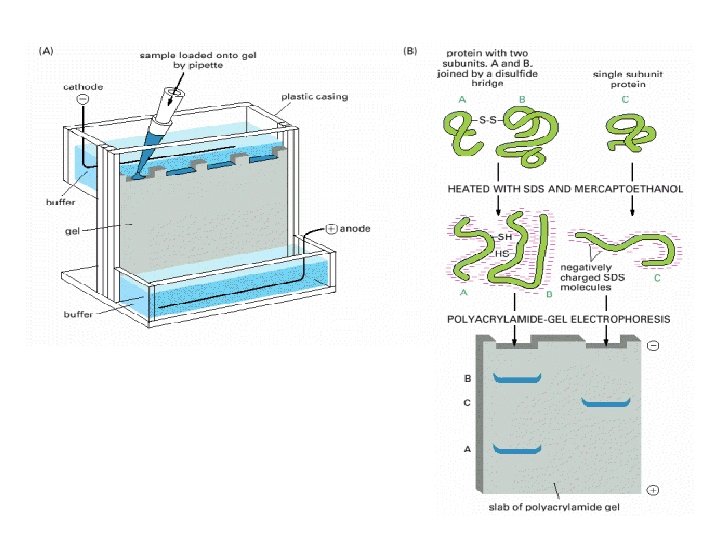
Use of Antigen Antibody Reaction • Primary Antibody Recognize & Binds Specifically to Certain Component (Antigen) within the Cell. • A secondary Antibody Recognize & binds Specifically to Primary antibody. • Location of Certain component can be seen. • Secondary Antibodies can be tagged with Fluorescent dyes or Enzyme End reaction To Produce a color.
Electrophoresis
- Slides: 41