STUDY ISLAND MCAS REVIEW Physical Science Assignment 7



- Slides: 12

STUDY ISLAND MCAS REVIEW Physical Science Assignment #7 Properties of Matter Elements, Compounds & Mixtures Physical & Chemical Changes

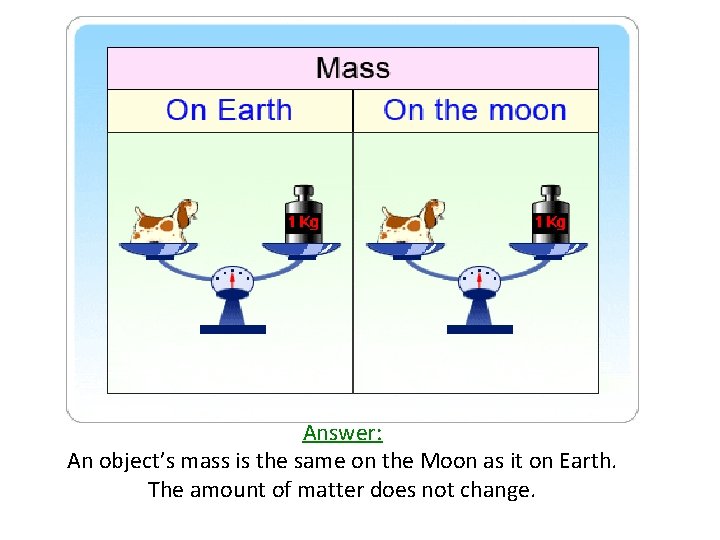
Matter • Matter is defined as anything that has mass and takes up space • Remember that mass is the total amount of material in an object CHALLENGE QUESTION: When an astronaut travels to the moon, does his/her mass change?

Answer: An object’s mass is the same on the Moon as it on Earth. The amount of matter does not change.

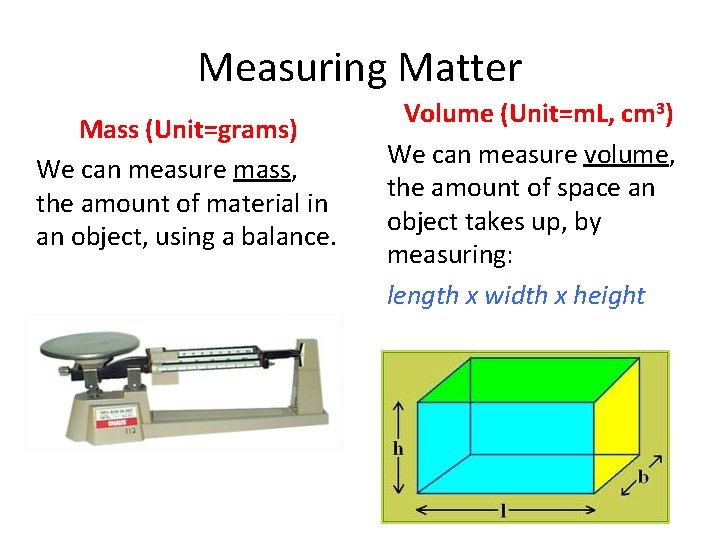
Measuring Matter Mass (Unit=grams) We can measure mass, the amount of material in an object, using a balance. Volume (Unit=m. L, cm 3) We can measure volume, the amount of space an object takes up, by measuring: length x width x height

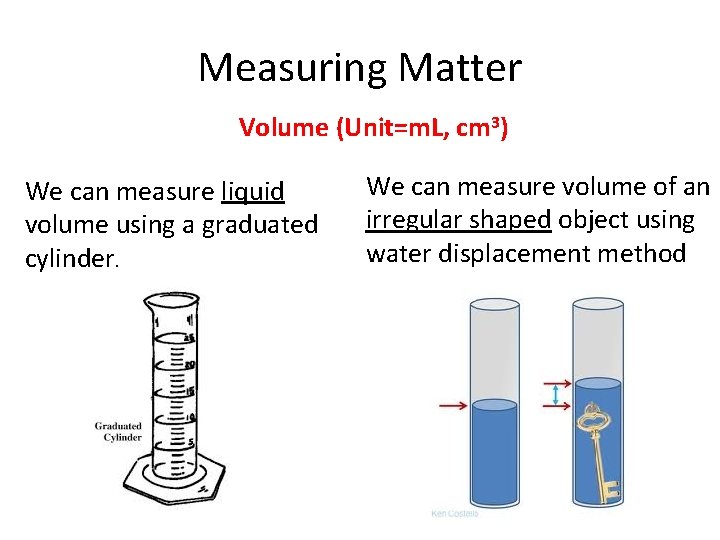
Measuring Matter Volume (Unit=m. L, cm 3) We can measure liquid volume using a graduated cylinder. We can measure volume of an irregular shaped object using water displacement method

Important Note About Matter • It is important to remember that almost everything is made up of matter and has mass, but ENERGY IS NOT MATTER • Therefore, energy such as heat, sound, and light has no mass or volume

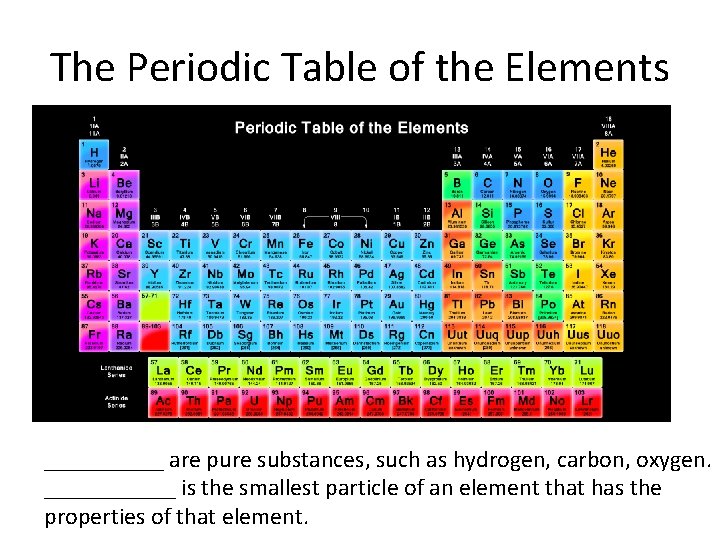
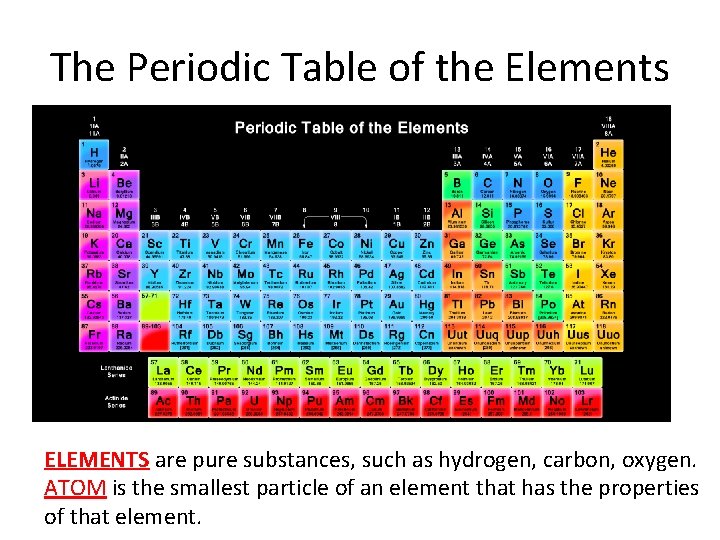
The Periodic Table of the Elements _____ are pure substances, such as hydrogen, carbon, oxygen. ______ is the smallest particle of an element that has the properties of that element.

The Periodic Table of the Elements ELEMENTS are pure substances, such as hydrogen, carbon, oxygen. ATOM is the smallest particle of an element that has the properties of that element.

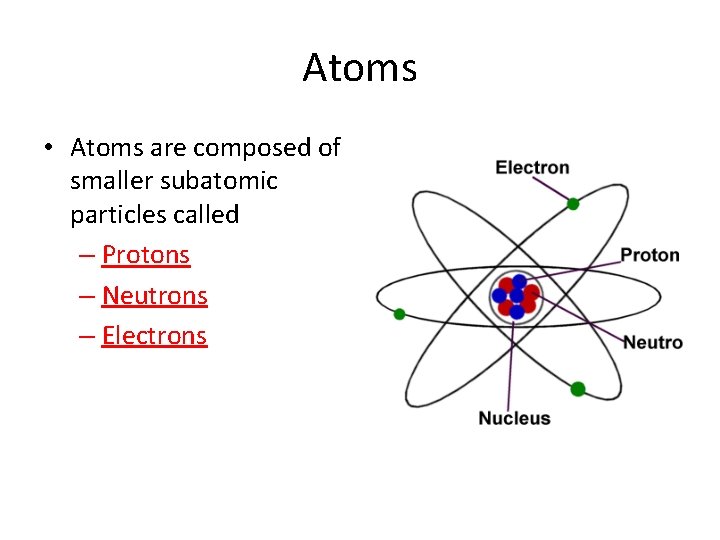
Atoms • Atoms are composed of smaller subatomic particles called – Protons – Neutrons – Electrons

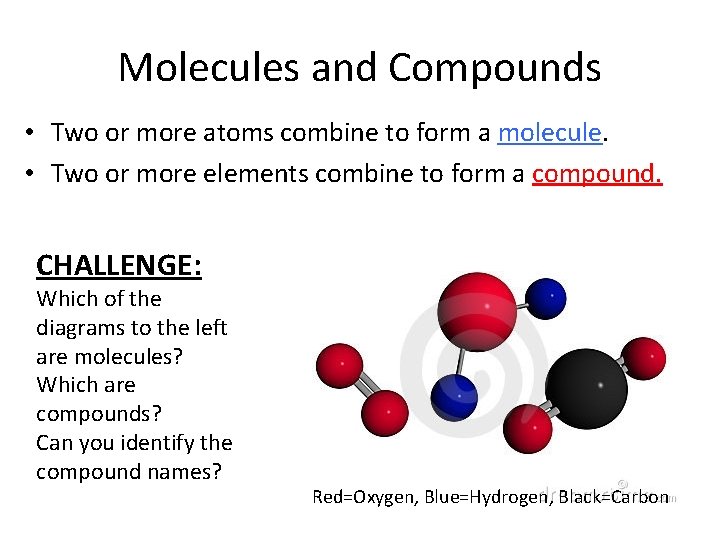
Molecules and Compounds • Two or more atoms combine to form a molecule. • Two or more elements combine to form a compound. CHALLENGE: Which of the diagrams to the left are molecules? Which are compounds? Can you identify the compound names? Red=Oxygen, Blue=Hydrogen, Black=Carbon

Physical Change A change of matter from one form to another without a change in chemical properties Chemical Change A change that occurs when one or more substances change into entirely new substances. Chemical changes cannot be reversed.

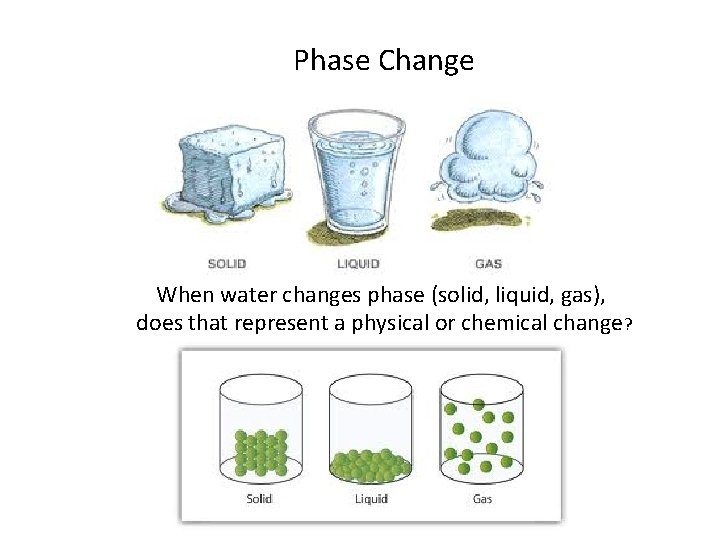
Phase Change When water changes phase (solid, liquid, gas), does that represent a physical or chemical change?