Study Designs in Epidemiology Dr Adchitre S A




















































































- Slides: 105

Study Designs in Epidemiology Dr Adchitre S A Associate Prof. Dept. of Community Medicine

What are study designs? � Structured approaches to address specific research questions. � Selection of research design is the single most important decision the investigator has to make. � Provide general guidelines for thinking about specific aspects of study conduct: ◦ sampling populations ◦ systematically collecting measurements ◦ analysing data � Strengths & limitations of specific designs are wellestablished

Why we conduct studies? � To estimate prevalence, incidence, proportion or mean value � To find out association between cause and effect � To see effect of any medicine, treatment or vaccine � To compare two diagnostic tests

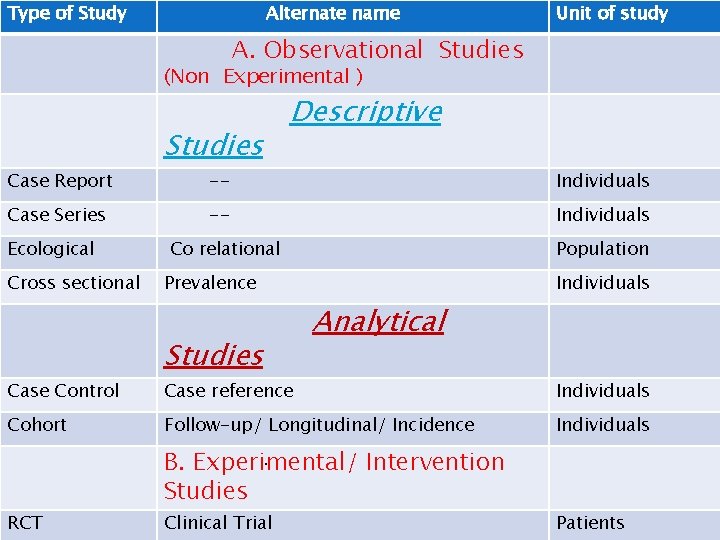
Type of Study Alternate name Unit of study A. Observational Studies (Non Experimental ) Case Report Case Series Ecological Cross sectional Case Control Cohort Studies Descriptive -- Individuals Co relational Population Prevalence Studies Individuals Analytical Case reference Individuals Follow-up/ Longitudinal/ Incidence Individuals . B. Experimental/ Intervention Studies RCT Clinical Trial Patients

Aim of Various Study designs: � 1. Descriptive Studies: to generate or Formulate hypothesis. � 2. � 3. Analytical Studies : to test hypothesis. Experimental Studies : to prove hypothesis.

DESCRIPTIVE EPIDEMIOLOGY �It describes �When-time distribution �Where-place distribution �Who-person distribution

PROCEDURES �Defining the population �Defining the disease under study �Describe the disease –time, place, person �Measurement of disease �Comparing with known indices �Formulation of hypothesis

PLACE DISTRIBUTION �International variation �National variation �Rural-urban �Local variation

TIME DISTRIBUTION �Short term fluctuations �a)common source epidemics �-single exposure or point source �-continuous or multiple exposure �b)Propogated epidemics �-person to person �-arthropod vector �-animal reservior �c)slow epidemics

� 2)Periodic fluctuations �-seasonal trend �-cyclic trend � 3)Long term or secular trends

PERSON DISTRIBUTION �Age �Sex �Ethnicity �Mariatal status �Occupation �Social class �Behaviour �Stress �Migration

Descriptive studies � Do not proceed to test a pre-formed hypothesis � Study is done only in one group of subjects; there is no comparison group � Can be community or hospital based � Study proceeds to describe: ◦ Mean value ◦ Proportion ◦ Distribution in person, place and time

Descriptive study designs 1) Case report : Brief, objective report of clinical characteristic or outcome from a single clinical subject or event � It is the first evidence of an unexpected or unusual event � Results are rarely generalizable

Descriptive study designs (Cont…) 2) Case report series : Objective and brief report of a clinical characteristic or outcome from a group of clinical subjects. � Problems in generalization due to – biased selection or un representativeness of subjects, lack of control group

3. Incidence - Prevalence studies Type of case series report… Entire population well defined and surveyed regarding parameters in question � Rates useful to compare populations obtained � Rates of little use in rare events � Sampling procedure if not random affects utility significantly

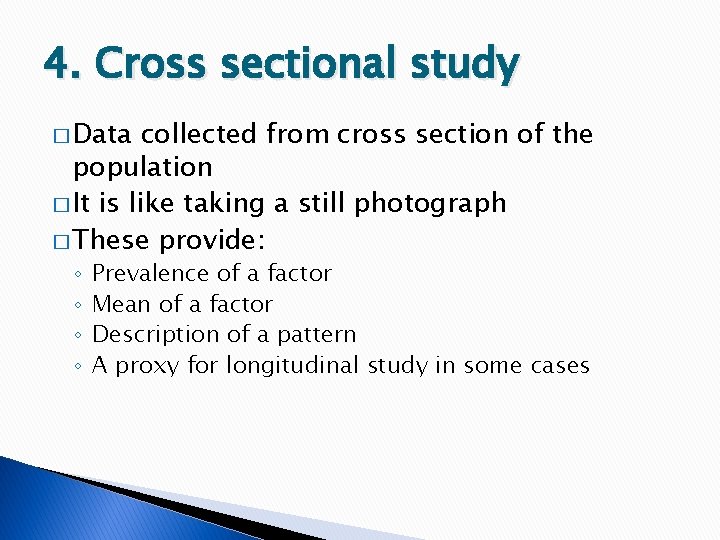
4. Cross sectional study � Data collected from cross section of the population � It is like taking a still photograph � These provide: ◦ ◦ Prevalence of a factor Mean of a factor Description of a pattern A proxy for longitudinal study in some cases

CRITERION PROSPECTIVE (COHORT) RETROSPECTIVE (CASE CONTROL) CROSS SECTIONAL COST AND TIME HIGH LOW SUITABLE FOR RARE ANTECEDENTS RARE OUTCOMES NONE SHOULD BE RARE RECALL LAPSE NOT LIKELY VERY LIKELY PRACTICALLY ABSENT LOSS OF INFORMATION HIGH CHANCE LOW CHANCE NO CHANCE ASSESSMENT OF CAUSE-EFFECT GOOD FAIR POOR EVALUATION OF RISK FACTORS GOOD FOR FAIR SINCE INCIDENCE OR EVALUATION RELATIVE RISK IS IN TERMS OF ODDS RATIO NONE, SINCE ONLY PREVALENCE CAN BE CALCULATED

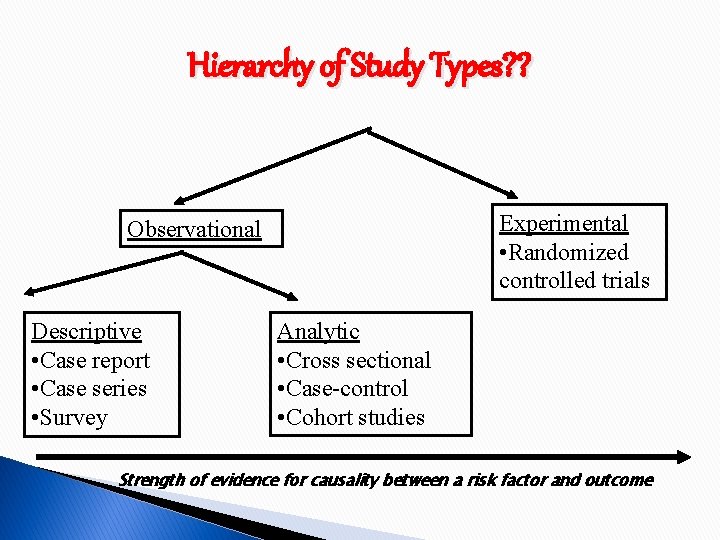
Hierarchy of Study Types? ? Experimental • Randomized controlled trials Observational Descriptive • Case report • Case series • Survey Analytic • Cross sectional • Case-control • Cohort studies Strength of evidence for causality between a risk factor and outcome

Timeframe of Studies �Prospective Study - looks forward, looks to the future, examines future events, follows a condition, concern or disease into the future time Study begins here

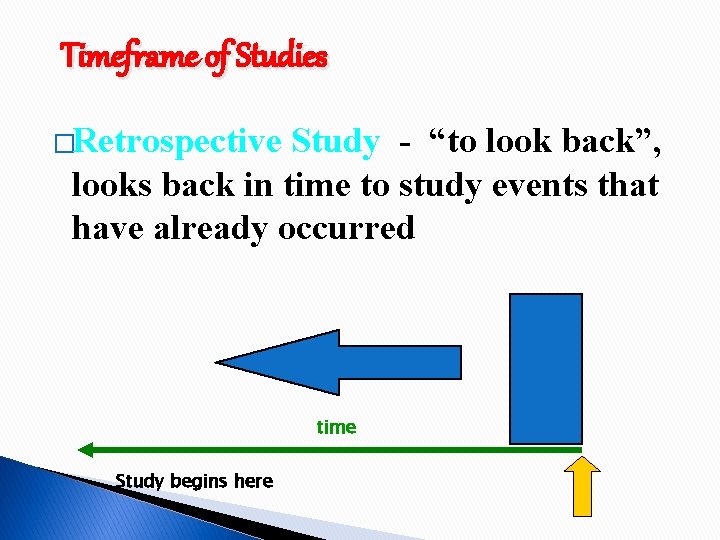
Timeframe of Studies �Retrospective Study - “to look back”, looks back in time to study events that have already occurred time Study begins here

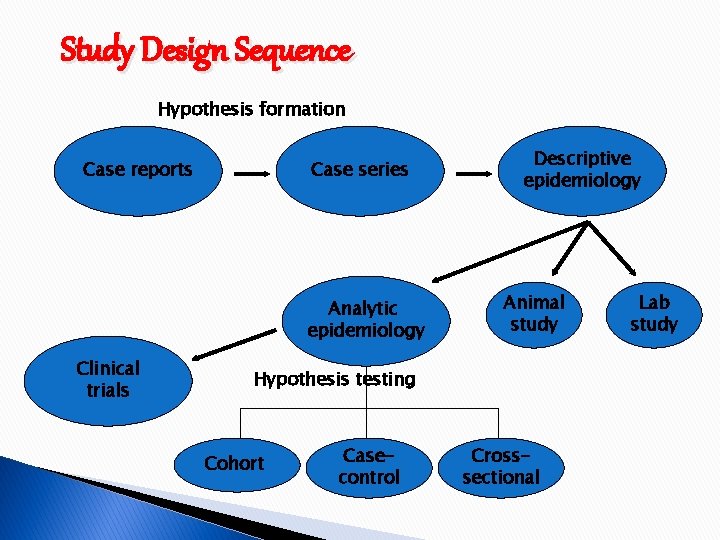
Study Design Sequence Hypothesis formation Case reports Case series Analytic epidemiology Clinical trials Descriptive epidemiology Animal study Hypothesis testing Cohort Casecontrol Crosssectional Lab study

ANALYTICAL EPIDEMIOLOGY Case- Control Study Cohort Study

Epidemiologic Study Designs �Case-Control Studies ◦ an “observational” design comparing exposures in disease cases vs. healthy controls from same population ◦ exposure data collected retrospectively ◦ most feasible design where disease outcomes are rare

Case-Control Studies Cases: Disease Controls: No disease

Retrospective study First approach to test the Causal hypothesis � Both Exposure & Outcome (Disease) have OCCURRED BEFORE the start of the study. � The � It Study Proceeds BACKWARDS from Effect To Cause. uses a CONTROL or Comparison Group to Support or Refute an Inference.

Basic steps � Selection of cases and control � Matching � Measurement of exposure � Analysis and interpretation

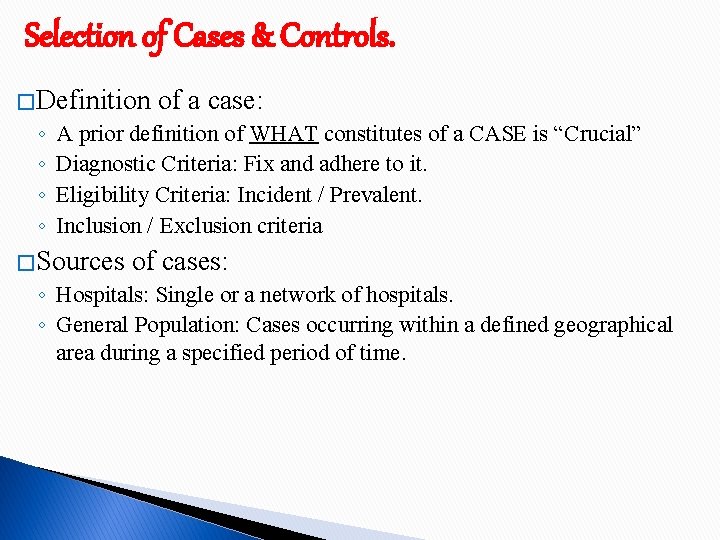
Selection of Cases & Controls. � Definition ◦ ◦ of a case: A prior definition of WHAT constitutes of a CASE is “Crucial” Diagnostic Criteria: Fix and adhere to it. Eligibility Criteria: Incident / Prevalent. Inclusion / Exclusion criteria � Sources of cases: ◦ Hospitals: Single or a network of hospitals. ◦ General Population: Cases occurring within a defined geographical area during a specified period of time.

Selection Of Controls: Most difficult � Must be FREE from the disease under study. � Similar to cases as possible EXCEPT for absence of the disease / factor under study. � Sources: ◦ ◦ Hospital Controls. Relatives. Neighborhood control. General Population. � How many controls : - 1: 1 (1: 4)

Matching �Controls may differ from Cases in many factors ( Age, Sex, occupation, Social status etc. ). � To ensure “COMPARIBALITY”: Matching. �“ A process by which we select controls in such a way that they are similar to cases with regard to certain PERTINENT variables which are known to influence the outcome of disease and which if not adequately matched for comparability could DISTORT or CONFOUND the results. ”


Measurement of Exposure � Information : Obtain Precisely in the same manner for both ( Cases & Controls). � Obtained by Interviews, questionnaires, studying past hospital / employment records, examinations. � Question of Bias or Error should be taken care of.

Case-Control Study-analysis A case-control study cannot yield estimates of incidence or prevalence of a disease, but it can provide descriptive information on the characteristics of the cases and an estimate of the strength of association between predictor variables and the presence or absence of disease.

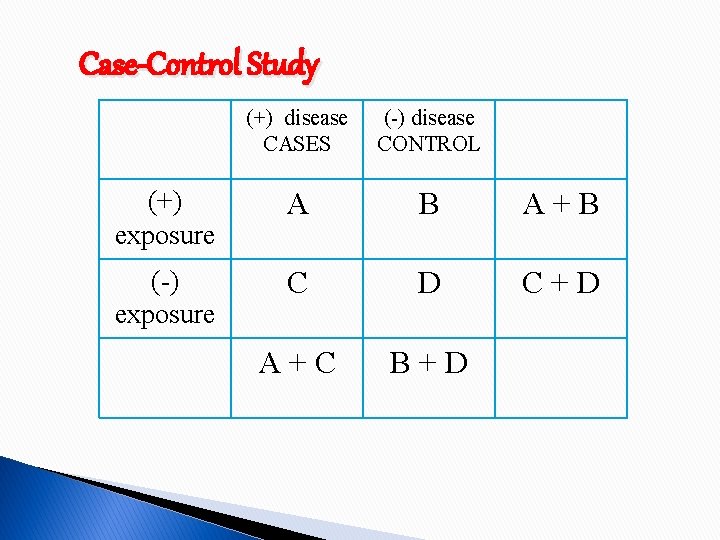
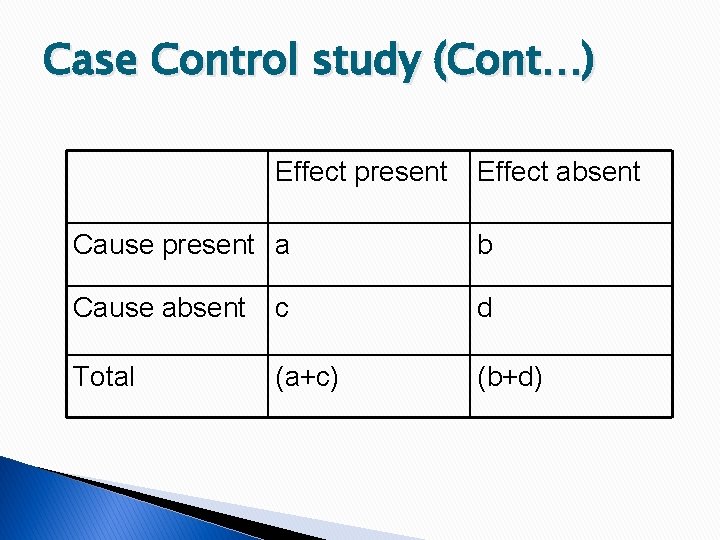
Case-Control Study (+) disease CASES (-) disease CONTROL (+) exposure A B A+B (-) exposure C D C+D A+C B+D

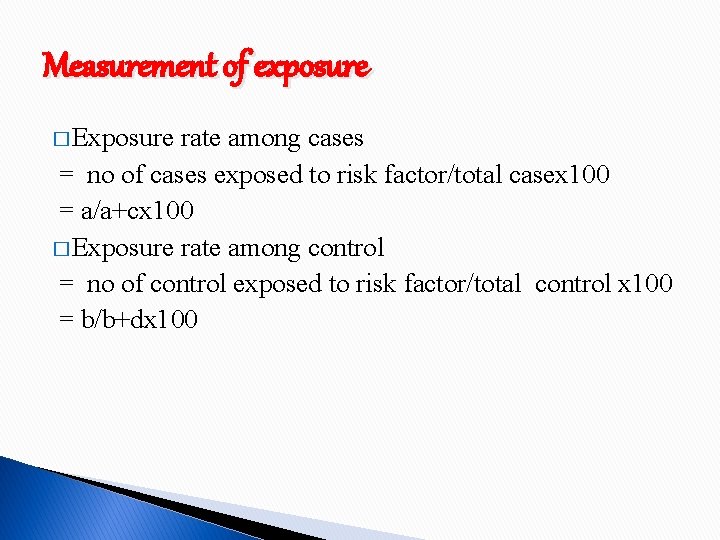
Measurement of exposure � Exposure rate among cases = no of cases exposed to risk factor/total casex 100 = a/a+cx 100 � Exposure rate among control = no of control exposed to risk factor/total control x 100 = b/b+dx 100

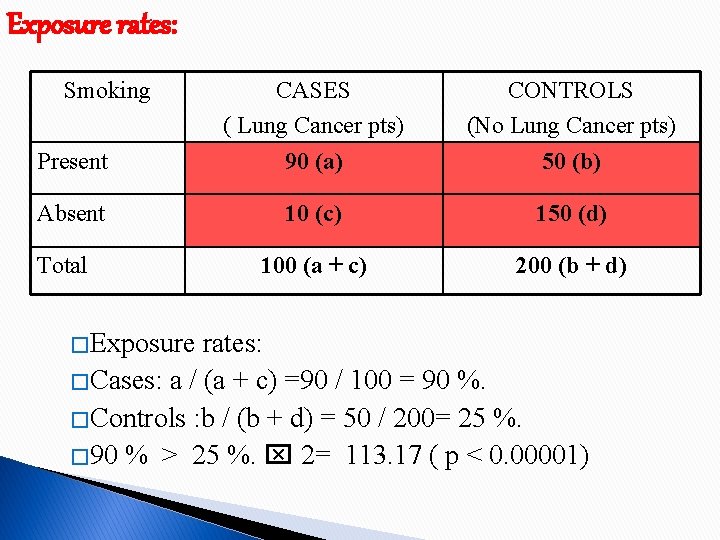
Exposure rates: Smoking Present CASES ( Lung Cancer pts) 90 (a) CONTROLS (No Lung Cancer pts) 50 (b) Absent 10 (c) 150 (d) 100 (a + c) 200 (b + d) Total � Exposure rates: � Cases: a / (a + c) =90 / 100 = 90 %. � Controls : b / (b + d) = 50 / 200= 25 %. � 90 % > 25 %. 2= 113. 17 ( p < 0. 00001)

Disease risk : Odds Ratio ( Strength of association ) � Cross product ratio. ◦ Disease investigated : relatively Rare. ◦ Cases : representative to those with the disease un general population. ◦ Controls : representative to those without the disease un general population. OR = ad/ bc = 90 * 150 / 10 * 50 = 27. Smokers showed a risk of having lung cancer 27 times that of Non-smokers.

Case - Control Study Interpretation of OR: � OR = 1: exposure is not related with disease � OR > 1: exposure is positively related with disease � OR < 1: exposure is negatively related with disease

BIAS IN CASE CONTROL STUDIES 1. 2. 3. 4. 5. Bias due to confounding Memory or recall bias Selection bias Berkesonian bias Interviewer’s bias

Advantages: � Relatively � Rapid easy to carry out. & Inexpensive. � Require comparatively few study subjects. � Particularly � No risk to study subjects. � Allows � Risk � No suitable to investigate RARE diseases. to study different aetiological factors can be identified. attrition problems. � Ethical problems minimal.

Disadvantages: �Bias (Memory of past records, accuracy uncertain, validation of information difficult. ) �Selection of appropriate CONTROL difficult. �We cannot measure incidence, & can only estimate the relative risk ( Odds ratio). �Do not distinguish between causes & associated factors. �Not suited for evaluation of therapy. �Major concern is representativeness of cases & controls.

Thalidomide - phocomelia

Thalidomide tragedy � First marketed as safe , non barbiturate hypnotic in Britain 1958 � 1961, at Congress of gynaecologist, attention was drawn to birth of large no. of babies born with congenital anomalies which was previously rare. � Thalidomide might be responsible � Out of 46 women with deformed babies 41 have+ve history compared with 300 controls without history � Later lab expt. Confirmed teratogenicity

Epidemiologic Study Designs �Cohort Studies ◦ an “observational” design comparing individuals with a known risk factor or exposure with others without the risk factor or exposure ◦ looking for a difference in the risk (incidence) of a disease over time ◦ best observational design ◦ data usually collected prospectively (some retrospective)

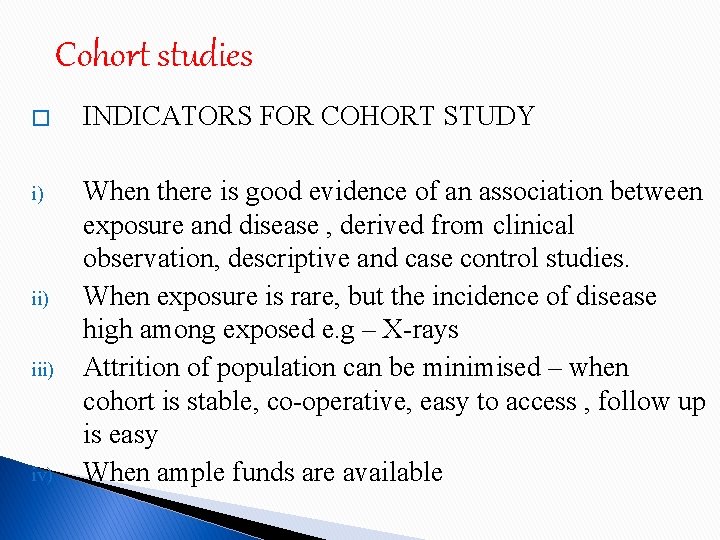
Cohort studies � INDICATORS FOR COHORT STUDY i) When there is good evidence of an association between exposure and disease , derived from clinical observation, descriptive and case control studies. When exposure is rare, but the incidence of disease high among exposed e. g – X-rays Attrition of population can be minimised – when cohort is stable, co-operative, easy to access , follow up is easy When ample funds are available ii) iv)

Prospective study First approach to test the Causal hypothesis � Cohorts are identified PRIOR to the appearance of the disease. � The Study groups are OBSERVED OVER A PERIOD OF TIME, to determine FREQUENY of Disease in them. � The study proceeds FORWARD from Cause to Effect.

General considerations �A group of people sharing common characteristics or experience within a defined time period. � Cohorts must be free from the disease under study. � Both the groups should be equally susceptible for the disease � Both the groups – comparable. � Diagnostic criteria defined beforehand

Timeframe of Studies �Prospective Study - looks forward, looks to the future, examines future events, follows a condition, concern or disease into the future time Study begins here

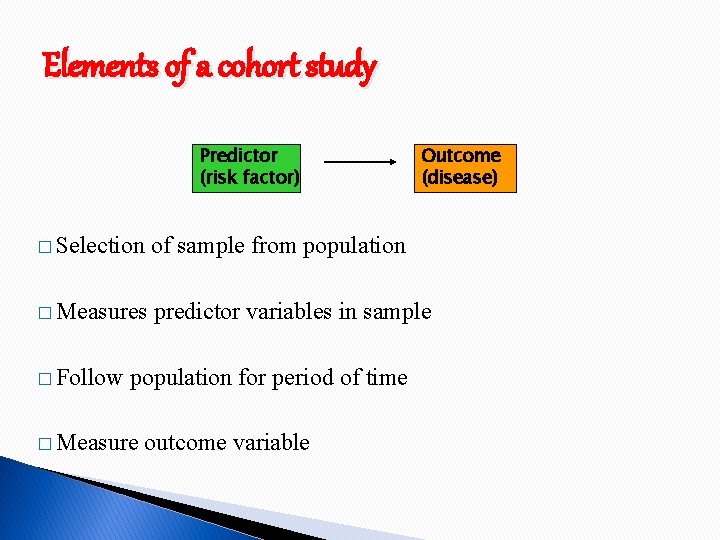
Elements of a cohort study Predictor (risk factor) Outcome (disease) � Selection of sample from population � Measures predictor variables in sample � Follow population for period of time � Measure outcome variable

Selection of study subjects. � General population. � Special groups. � Exposure groups. Obtaining data on Exposure. Ø Cohort members interview. Ø Review of records. Ø Medical examinations. Ø Environmental surveys.

Selection of control groups. � Internal comparison. � External Comparison. � Comparison with general population rates. Follow-up. �Periodic medical examination. �Reviewing physician & hospital records. �Routine surveillance of death records. �Mailed questionnaires, telephone calls, periodic home visits. (Preferably all three on ANNUAL basis. )

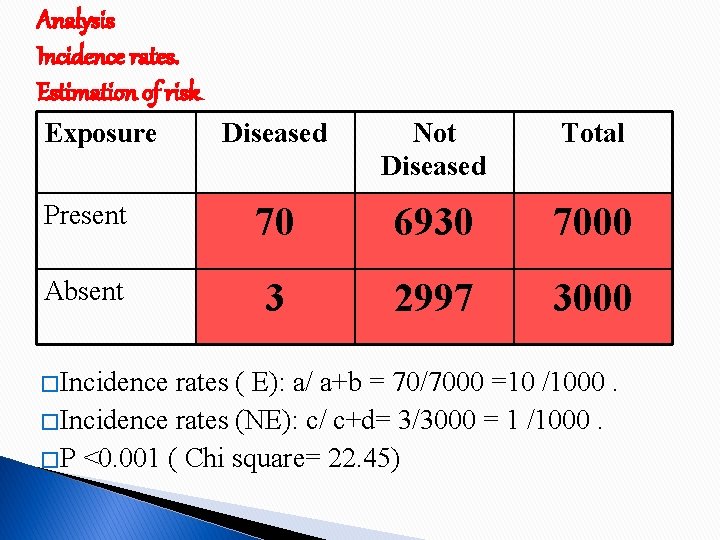
Analysis Incidence rates. Estimation of risk Exposure Diseased Not Diseased Total Present 70 6930 7000 Absent 3 2997 3000 � Incidence rates ( E): a/ a+b = 70/7000 =10 /1000. � Incidence rates (NE): c/ c+d= 3/3000 = 1 /1000. � P <0. 001 ( Chi square= 22. 45)

Relative Risk Incidence among Exposed � RR = --------------------Incidence among Not Exposed = 10/1 = 10 Smokers are 10 times at greater risk of developing Lung cancer than non smokers

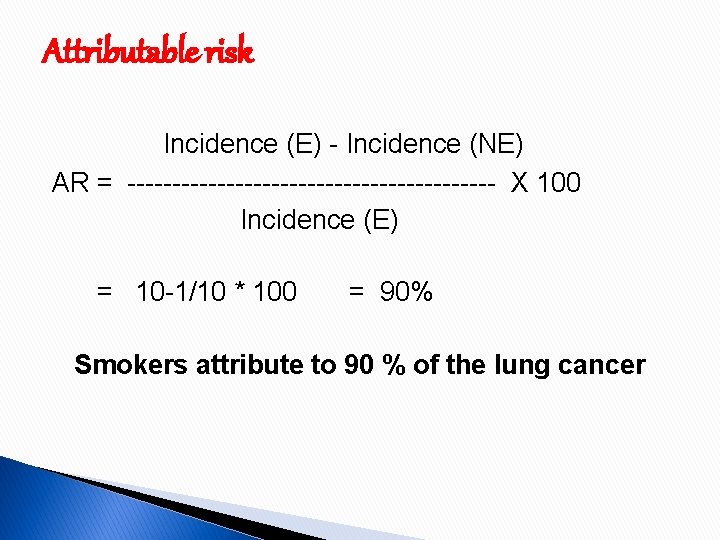
Attributable risk Incidence (E) - Incidence (NE) AR = --------------------- X 100 Incidence (E) = 10 -1/10 * 100 = 90% Smokers attribute to 90 % of the lung cancer

ADVANTAGES i) Incidence can be calculated ii) Several possible outcomes related to exposure can be studied iii) Provides a direct estimates of relative risk iv) Dose – response ratios can be calculated v) Since comparison groups are formed before disease develops, certain forms of bias can be minimised like.

DISADVANTAGES i) Involves large no. of population ii) Long time to complete the study and obtain results iii) Administrative problems iv) Usual to lose some part of cohort v) Selection of comparison group vi) Changes in standards vii) Study itself may alter the peoples behavior

Experimental epidemiology � Randomised controlled trials � Non randomized controlled trials

Some study designs (Cont…) 6) Randomized controlled trials : This is an experimental study � Like cohort study (except intervention) � Faces additional problems of cost, ethics and feasibility � Steps : Preparing a protocol, Selection of reference and experimental populations, Randomization, Intervention, Follow up, Assessment of outcome and analysis � Analysis : Rates of measured outcomes are compared

Purpose � To provide scientific proof of aetiological or risk factors which may permit modification or control of the disease � To provide a method of measuring the effectiveness and efficiency of health services for the prevention, control and treatment of disease and improve the health of the community

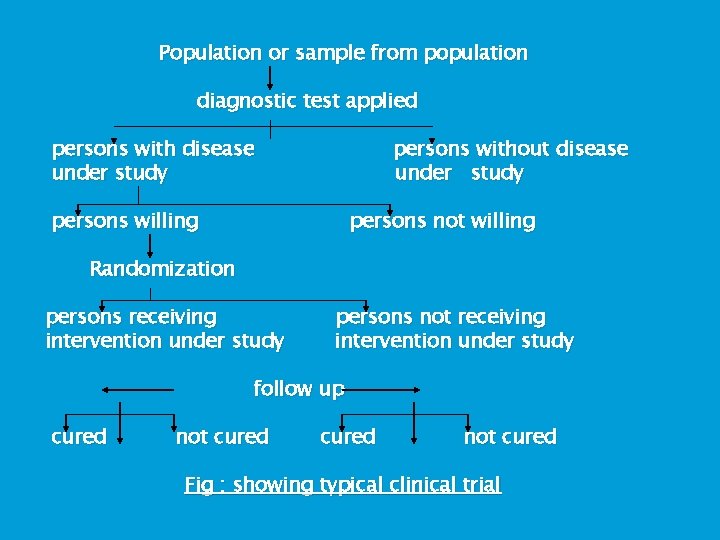
Population or sample from population diagnostic test applied persons with disease under study persons without disease under study persons willing persons not willing Randomization persons receiving intervention under study persons not receiving intervention under study follow up cured not cured Fig : showing typical clinical trial

Consent? !

RANDOMISED CONTROLLED TRIALS �STEPS � 1)drawing up a protocol-prevents bias �-aims & obj �-criteria for selection-study n control grp �-sample size �-intervention procedures

� 2)selection of reference & experimental population �-reference pop-city, school, industry �-expt/study pop-from ref pop � -participates in study � -random selection �Volunteers-informed concent, representative of the sample, qualified or eligible for trial

� 3) randomization-eliminates bias �Study & control group �comparability –like with like �Table of random no is used � 4)manipulation �-intervene study grp-drug, vaccine , diet �-independent variables: dependent variables

� 5)follow up �-expt & control grp �-till final assessment of outcome �-lost to follow up-death, migration � 6)assessment �-final outcome �-positive results �-negative results

Some study designs � Concurrent parallel study design: Patients remain in the study group or control group for the complete duration of the investigation � Cross over type of study design: After taking treatment the study and control groups are taken off from the medication. Then two groups are switched, those receiving treatment receive placebo and controls receive treatment

Types of RCT � Clinical trial � Preventive trials � Risk factor trials � Cessation experiments � Trial of aetiological agents � Evaluation of health services

Phases of clinical trial � Pharmaceutical clinical trials are commonly classified into four phases � Phase I Usually a small group(20 -40)of healthy volunteers selected. Includes trials designed to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of a therapy There are 2 specific kinds of phase I trials. SAD studies& MAD studies: single ascending dose , multiple ascending dose


Know more about RCT World Health Organization http: //www. who. int for “ the International Clinical Trials Registry Platform” National Institutes of Health http: //www. clinicaltrials. gov http: //www. nih. gov National Cancer Institute http: //www. cancer. gov Centers for Disease Control and Prevention http: //www. cdc. gov

Thank You !!!!!

STATUTARY WARNING!

Research: Ground reality? !




Types of scientific studies � Non experimental (Observational studies) � Investigator has no control over major independent or predictor variables � Existing phenomenon used � Experimental studies � Investigator has control over major independent and predictor variables � Study tests the effect of intervention on certain aspect of health or disease

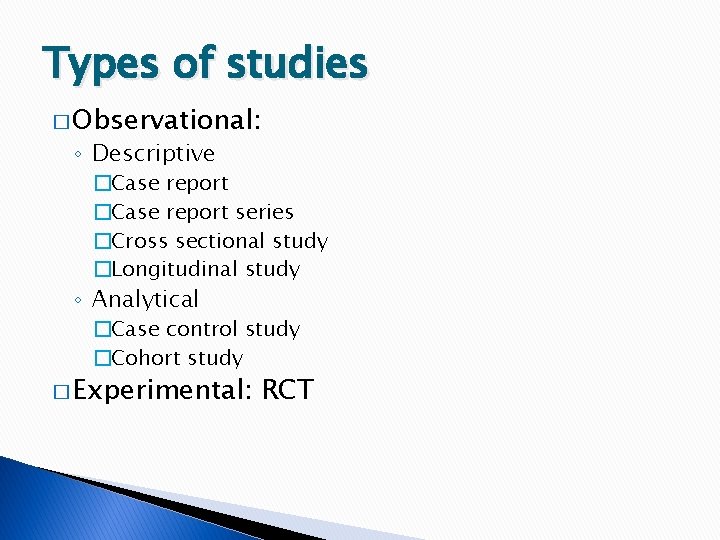
Types of studies � Observational: ◦ Descriptive �Case report series �Cross sectional study �Longitudinal study ◦ Analytical �Case control study �Cohort study � Experimental: RCT

Description is about… � Person: age, sex, race, religion, caste, social class, ethnicity, education, occupation, income, marital status, health status, smoking status, personality � Place: International, regional, national, institutional, local, rural/urban � Time: epidemic occurrence, time trends, secular, cyclic, seasonal � Family: birth order, parity, family size, maternal age, birth interval, family type

Cross sectional study…(Cont) � Besides describing a disease in terms of time, place and person the end point can bedeveloping a hypothesis � Help in assessing load of disease, needs, resources, patterns of health utilization, practices and knowledge-attitudes � Help in identifying target groups

5. Longitudinal descriptive study � It follows a single group of subjects over a defined period of time � More scientific than cross sectional study but more costly and more time consuming � We can find out: incidence of a disease, describe natural history of a disease, describe health related natural phenomena, study trend of a disease or health related phenomena

Steps of a descriptive study � Write down research question and its significance � Enlist variables you want to study (related to time, place, person, family etc) � Specify the scales of measurement � Define the total and actual population � Calculate sample size and specify the sampling procedure

Steps of a descriptive study…(Cont) � Explicitly state inclusion and exclusion criteria � Give a detailed thought to your method of measurement and the way to avoid bias or ensure validity and reliability � Conduct pilot study � Data collection � Organize data � Analyze data � Draw conclusions � Prepare a report and generate a hypothesis

THANK YOU !

4) Case control study : Common first approach to test causal hypothesis � Exposure and outcome have occurred before start of study � Proceeds backwards from effect to cause � Uses control group to support or refute inference � Basic steps : selection of cases and controls, matching, measurement of exposure and analysis and interpretation � Odds ratio obtained

Case Control study 1) Selection of cases: Define case by using diagnostic criteria and eligibility criteria Cases can be from hospitals or general population Controls must be free from disease under question Can be obtained from hospital, relatives, neighborhood, general population One control for one case; maximum 4

Case Control study (Cont…) � Confounding factors and role of matching It is a factor that is one which is associated with “exposure” but is itself a “risk factor” for the disease Alcohol--- esophageal cancer study smoking is confounding factor � Group and paired matching

Case Control study (Cont…) Effect present Effect absent Cause present a b Cause absent c d Total (a+c) (b+d)

Case Control study (Cont…) � Exposure rates Cases = a/(a+c) Controls= b/(b+d) Odds Ratio= ad/bc If it is 6, it means risk of getting disease in exposed it 6 times than that of non exposed

JUST RELAX A WHILE !





Some study designs (Cont…) 5) Cohort study : Used to obtain additional evidence to support or refute a hypothesis � Study goes from cause to effect � Uses control cohort to support or refute inference � Involves a period of observation or follow up � Steps are: selection of study subjects, obtaining data on exposure, selection of comparison group, follow up, analysis and interpretation � Relative risk, attributable risk and population attributable risk obtained

Cohort study (Cont…) � Cohort is defined as a group of people who share a common characteristic or exposure within a specified time period � Cohort studies are indicated when there is good evidence of association between exposure and disease and when exposure is rare but the incidence of disease in exposed is high

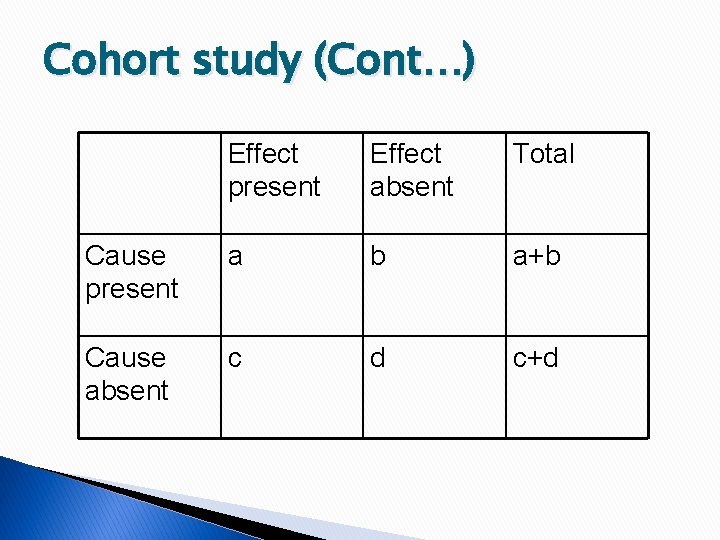
Cohort study (Cont…) Effect present Effect absent Total Cause present a b a+b Cause absent c d c+d

Cohort study (Cont…) � Relative risk = incidence among exposed/ incidence in non exposed � Attributable risk = (Incidence of disease among exposed – incidence among non exposed)/ incidence rate among exposed � Population attributable risk = Incidence of disease in the total population – incidence of disease among those who are not exposed to cause

Phases of clinical trial (Cont…) � Phase II Once the safety of therapy has been confirmed in phase I trials, phase II trials are performed on larger groups(200 -300) and are designed to assess clinical efficacy of therapy; as well as to continue phase I assessments in a larger group of volunteers and patients. The development process for a new drug commonly fails during phase II trials due to the discovery of poor efficacy or toxic effects.

Phases of clinical trial (Cont…) � Phase III studies are randomized controlled trials on large patient groups (300 -3000 or more) and are aimed at being the definitive assessment of the new therapy. Once the drug has proven satisfactory over phase III trials, the trial results are usually combined into a large document containing a comprehensive description of the methods and results of human and animal studies, manufacturing procedures, formulation details, and shelf life. This ‘regulatory submission’ information is provided for review to various regulatory authorities for marketing approval.

An uphill task!

Phases of clinical trial (Cont…) � Phase IV : Phase IV trials involve post-launch safety surveillance and ongoing technical support of drug. It is designed to detect any rare or long term adverse effects over a much larger patient population and time scale than was possible during the initial clinical trials. Such adverse effects detected by phase-IV trials may result in the withdrawal or restriction

Some study designs (Cont…) � 7) Meta Analysis : Statistical analysis combining results of several independent studies considered combinable by the researcher. � It is like a review of articles but in contrast to it, it attempts to analyze statistically aggregate results to derive an integrated conclusion � Faces problems due to – publication bias and varying quality of studies

I hope I haven’t done this to you!


EPIDEMIOLOGY DEFINITION