Studies AHEAD COSMOS and COMPASS The AHEAD Study






- Slides: 67

Studies AHEAD COSMOS and COMPASS

The AHEAD Study

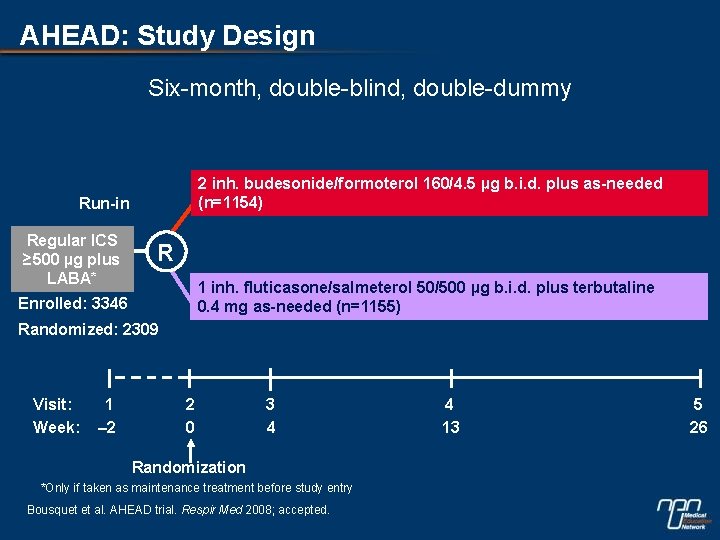
AHEAD: Study Design Six-month, double-blind, double-dummy 2 inh. budesonide/formoterol 160/4. 5 µg b. i. d. plus as-needed (n=1154) Run-in Regular ICS ≥ 500 µg plus LABA* R 1 inh. fluticasone/salmeterol 50/500 µg b. i. d. plus terbutaline 0. 4 mg as-needed (n=1155) Enrolled: 3346 Randomized: 2309 Visit: Week: 1 – 2 2 0 3 4 Randomization *Only if taken as maintenance treatment before study entry Bousquet et al. AHEAD trial. Respir Med 2008; accepted. 4 13 5 26

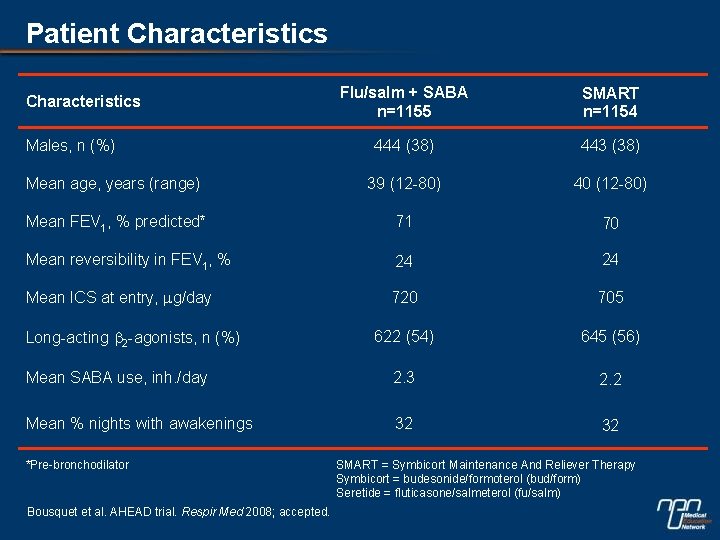
Patient Characteristics Flu/salm + SABA n=1155 SMART n=1154 444 (38) 443 (38) Mean age, years (range) 39 (12 -80) 40 (12 -80) Mean FEV 1, % predicted* 71 70 Mean reversibility in FEV 1, % 24 24 Mean ICS at entry, mg/day 720 705 622 (54) 645 (56) Mean SABA use, inh. /day 2. 3 2. 2 Mean % nights with awakenings 32 32 Characteristics Males, n (%) Long-acting b 2 -agonists, n (%) *Pre-bronchodilator Bousquet et al. AHEAD trial. Respir Med 2008; accepted. SMART = Symbicort Maintenance And Reliever Therapy Symbicort = budesonide/formoterol (bud/form) Seretide = fluticasone/salmeterol (fu/salm)

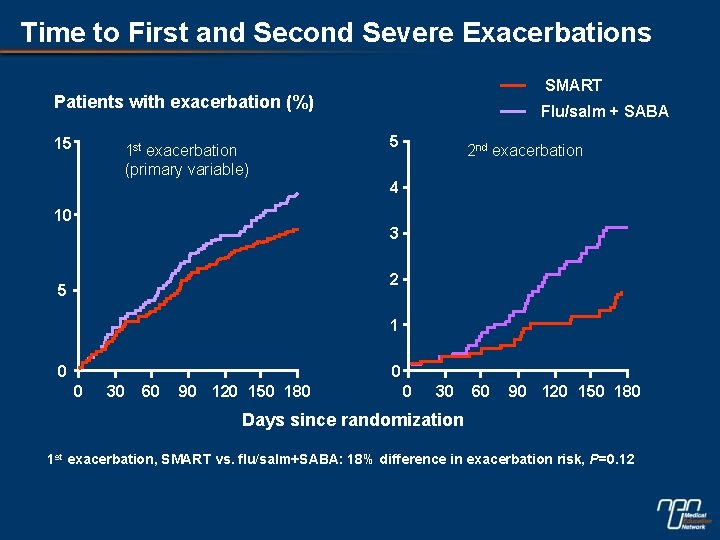
Time to First and Second Severe Exacerbations SMART Patients with exacerbation (%) 15 1 st exacerbation (primary variable) Flu/salm + SABA 5 2 nd exacerbation 4 10 3 2 5 1 0 0 0 30 60 90 120 150 180 Days since randomization 1 st exacerbation, SMART vs. flu/salm+SABA: 18% difference in exacerbation risk, P=0. 12

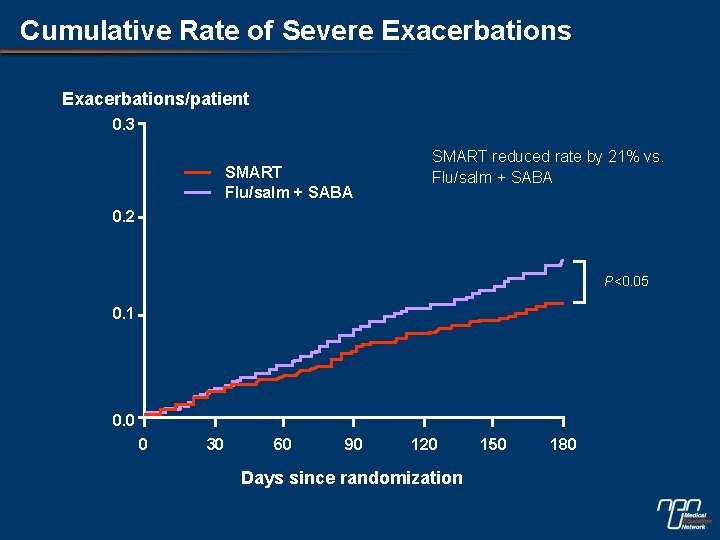
Cumulative Rate of Severe Exacerbations/patient 0. 3 SMART Flu/salm + SABA SMART reduced rate by 21% vs. Flu/salm + SABA 0. 2 P<0. 05 0. 1 0. 0 0 30 60 90 120 Days since randomization 150 180

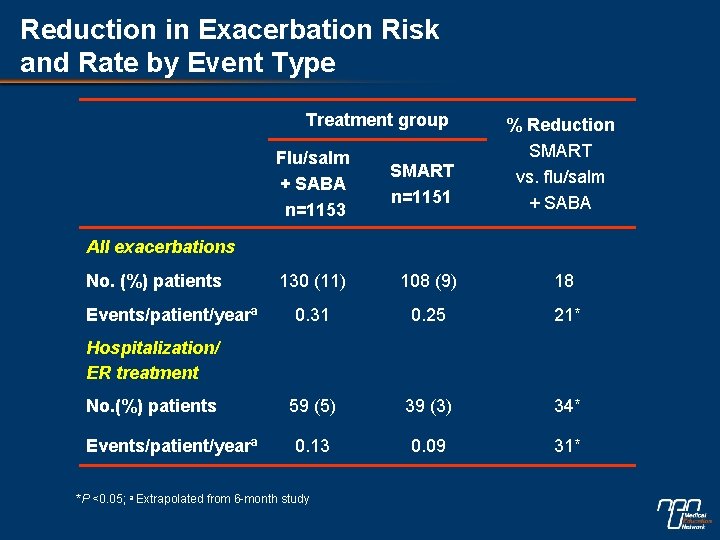
Reduction in Exacerbation Risk and Rate by Event Type Treatment group % Reduction SMART vs. flu/salm + SABA Flu/salm + SABA n=1153 SMART n=1151 130 (11) 108 (9) 18 0. 31 0. 25 21* 59 (5) 39 (3) 34* 0. 13 0. 09 31* All exacerbations No. (%) patients Events/patient/yearª Hospitalization/ ER treatment No. (%) patients Events/patient/yearª *P <0. 05; a Extrapolated from 6 -month study

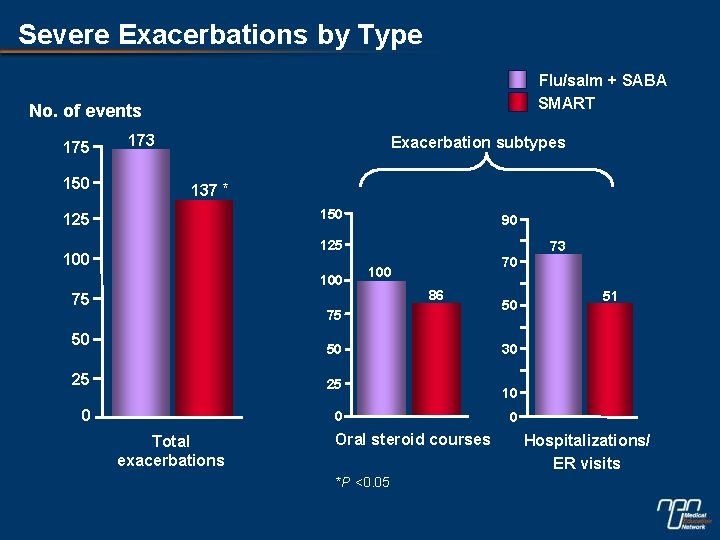
Severe Exacerbations by Type Flu/salm + SABA SMART No. of events 175 150 173 Exacerbation subtypes 137 * 150 125 90 125 100 75 73 70 100 86 75 50 50 25 25 0 0 Total exacerbations Oral steroid courses *P <0. 05 50 51 30 10 0 Hospitalizations/ ER visits

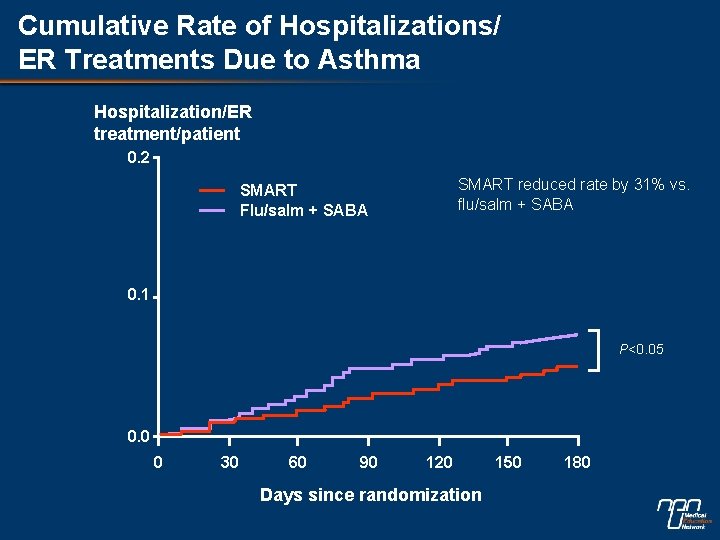
Cumulative Rate of Hospitalizations/ ER Treatments Due to Asthma Hospitalization/ER treatment/patient 0. 2 SMART reduced rate by 31% vs. flu/salm + SABA SMART Flu/salm + SABA 0. 1 P<0. 05 0. 0 0 30 60 90 120 Days since randomization 150 180

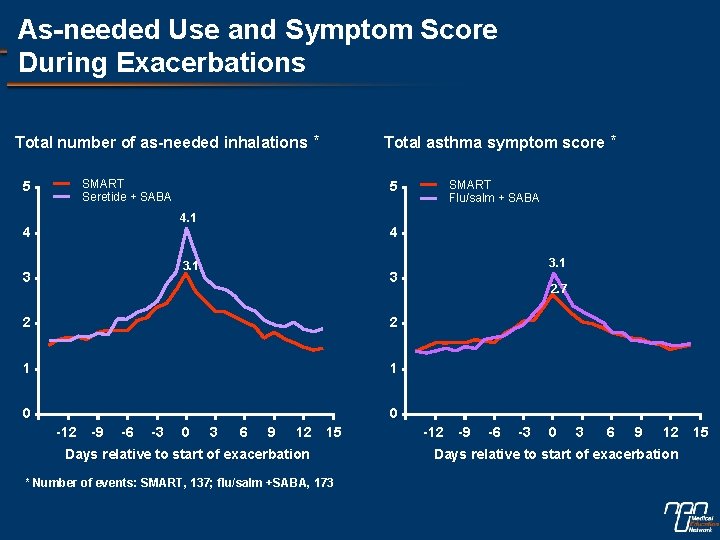
As-needed Use and Symptom Score During Exacerbations Total number of as-needed inhalations * SMART Seretide + SABA 5 4 3. 1 3 3 2 2 1 1 0 0 -12 -9 -6 -3 SMART Flu/salm + SABA 5 4. 1 4 Total asthma symptom score * 0 3 6 9 12 15 Days relative to start of exacerbation * Number of events: SMART, 137; flu/salm +SABA, 173 2. 7 -12 -9 -6 -3 0 3 6 9 12 15 Days relative to start of exacerbation

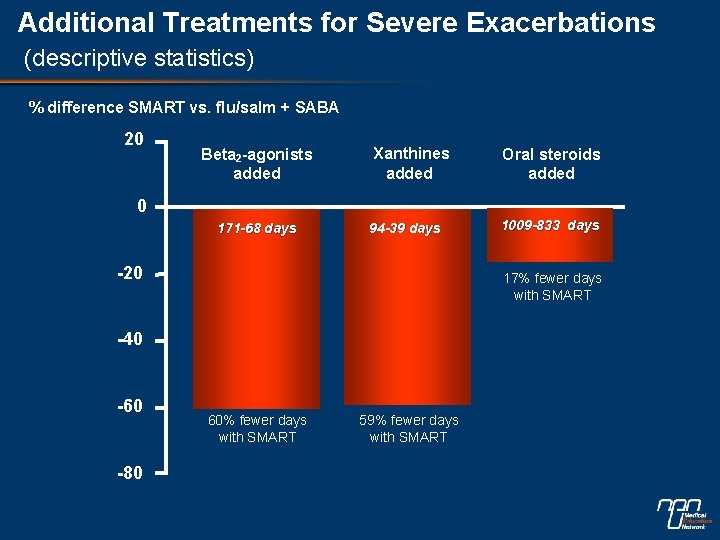
Additional Treatments for Severe Exacerbations (descriptive statistics) % difference SMART vs. flu/salm + SABA 20 Beta 2 -agonists added Xanthines added Oral steroids added 0 171 -68 days 94 -39 days -20 17% fewer days with SMART -40 -60 -80 1009 -833 days 60% fewer days with SMART 59% fewer days with SMART

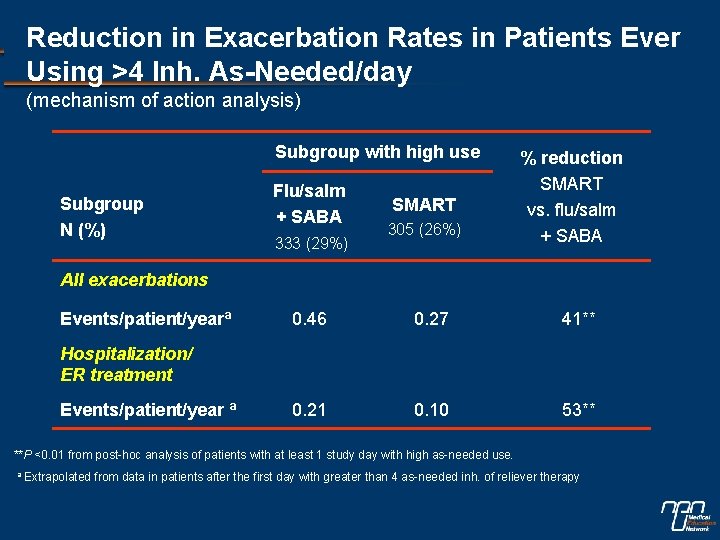
Reduction in Exacerbation Rates in Patients Ever Using >4 Inh. As-Needed/day (mechanism of action analysis) Subgroup with high use Subgroup N (%) Flu/salm + SABA 333 (29%) SMART 305 (26%) % reduction SMART vs. flu/salm + SABA All exacerbations Events/patient/yearª 0. 46 0. 27 41** 0. 21 0. 10 53** Hospitalization/ ER treatment Events/patient/year ª **P <0. 01 from post-hoc analysis of patients with at least 1 study day with high as-needed use. a Extrapolated from data in patients after the first day with greater than 4 as-needed inh. of reliever therapy

Change in Awakenings Due to Asthma Awakenings (%) NS 40 20 0 Run-in Treatment Flu/salm + SABA Run-in Treatment SMART

Change in As-needed Inhalation-free Days As-needed free days (%) NS 60 40 20 0 Run-in Treatment Flu/salm + SABA Run-in Treatment SMART

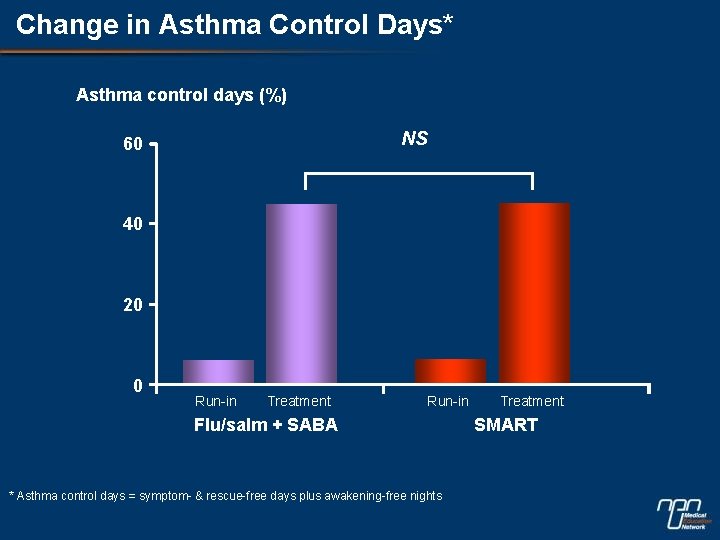
Change in Asthma Control Days* Asthma control days (%) NS 60 40 20 0 Run-in Treatment Run-in Flu/salm + SABA * Asthma control days = symptom- & rescue-free days plus awakening-free nights Treatment SMART

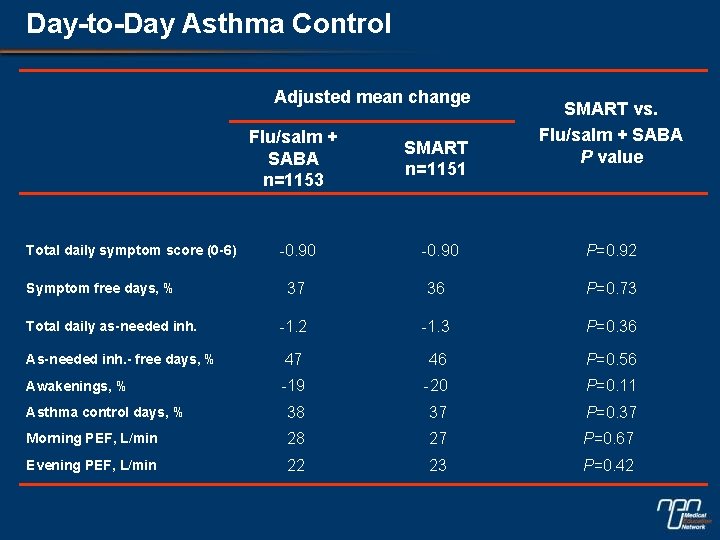
Day-to-Day Asthma Control Adjusted mean change SMART vs. Flu/salm + SABA P value Flu/salm + SABA n=1153 SMART n=1151 -0. 90 P=0. 92 37 36 P=0. 73 -1. 2 -1. 3 P=0. 36 As-needed inh. - free days, % 47 46 P=0. 56 Awakenings, % -19 -20 P=0. 11 Asthma control days, % 38 37 P=0. 37 Morning PEF, L/min 28 27 P=0. 67 Evening PEF, L/min 22 23 P=0. 42 Total daily symptom score (0 -6) Symptom free days, % Total daily as-needed inh.

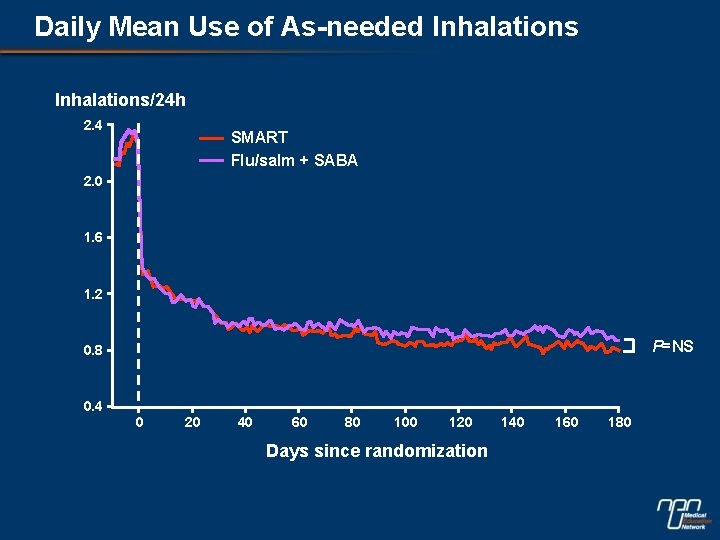
Daily Mean Use of As-needed Inhalations/24 h 2. 4 SMART Flu/salm + SABA 2. 0 1. 6 1. 2 P=NS 0. 8 0. 4 0 20 40 60 80 100 120 Days since randomization 140 160 180

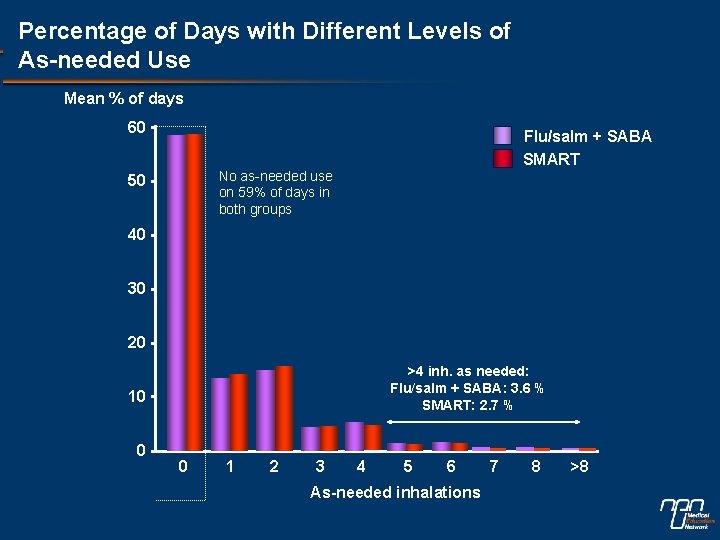
Percentage of Days with Different Levels of As-needed Use Mean % of days 60 Flu/salm + SABA SMART No as-needed use on 59% of days in both groups 50 40 30 20 >4 inh. as needed: Flu/salm + SABA: 3. 6 % SMART: 2. 7 % 10 0 0 1 2 3 4 5 6 As-needed inhalations 7 8 >8

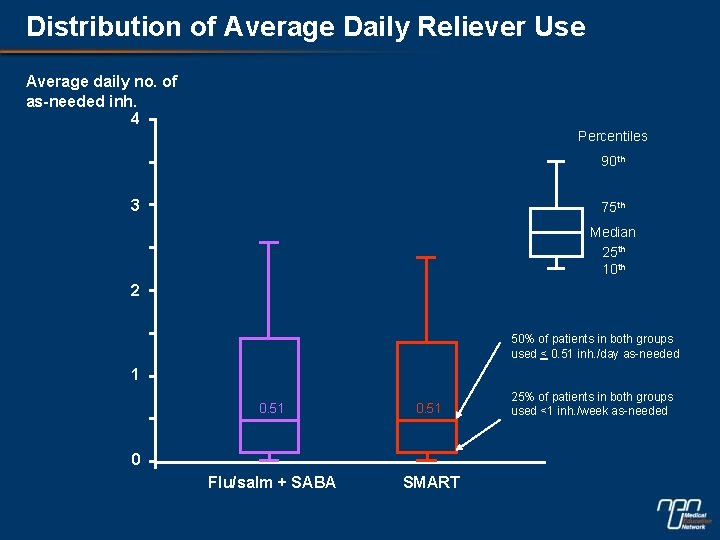
Distribution of Average Daily Reliever Use Average daily no. of as-needed inh. 4 Percentiles 90 th 3 75 th Median 25 th 10 th 2 50% of patients in both groups used < 0. 51 inh. /day as-needed 1 0. 51 Flu/salm + SABA SMART 0 25% of patients in both groups used <1 inh. /week as-needed

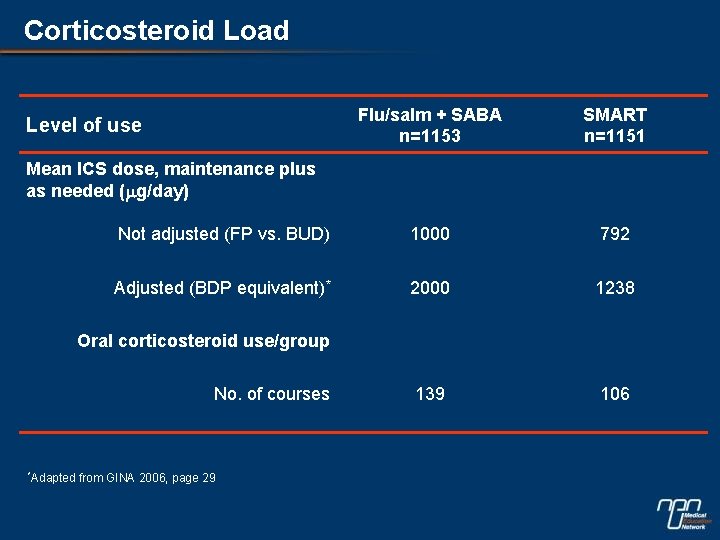
Corticosteroid Load Flu/salm + SABA n=1153 SMART n=1151 Not adjusted (FP vs. BUD) 1000 792 Adjusted (BDP equivalent)* 2000 1238 139 106 Level of use Mean ICS dose, maintenance plus as needed (mg/day) Oral corticosteroid use/group No. of courses *Adapted from GINA 2006, page 29

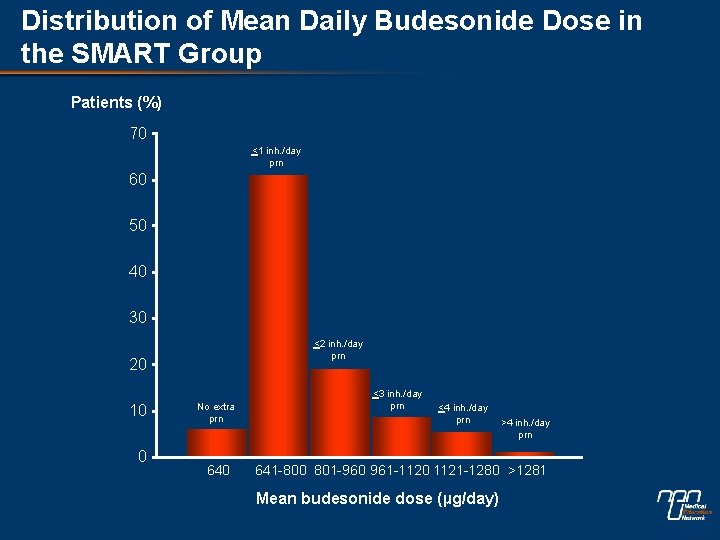
Distribution of Mean Daily Budesonide Dose in the SMART Group Patients (%) 70 <1 inh. /day prn 60 50 40 30 <2 inh. /day prn 20 10 0 No extra prn 640 <3 inh. /day prn <4 inh. /day prn >4 inh. /day prn 641 -800 801 -960 961 -1120 1121 -1280 >1281 Mean budesonide dose (µg/day)

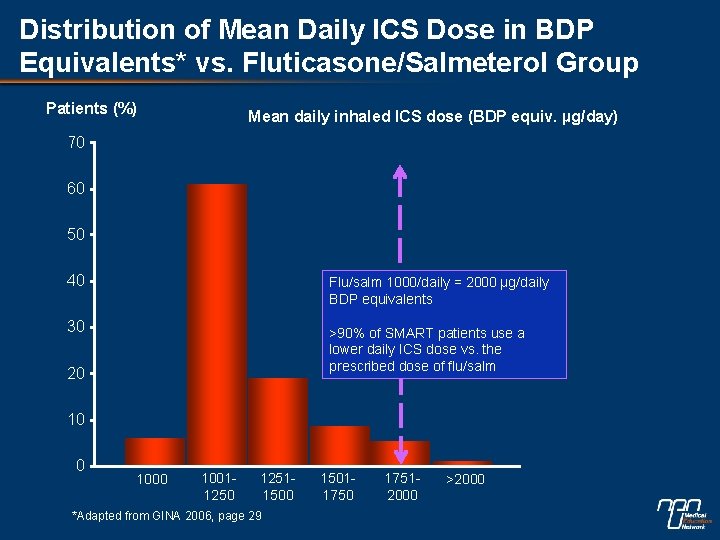
Distribution of Mean Daily ICS Dose in BDP Equivalents* vs. Fluticasone/Salmeterol Group Patients (%) Mean daily inhaled ICS dose (BDP equiv. µg/day) 70 60 50 40 Flu/salm 1000/daily = 2000 µg/daily BDP equivalents 30 >90% of SMART patients use a lower daily ICS dose vs. the prescribed dose of flu/salm 20 10011250 12511500 *Adapted from GINA 2006, page 29 15011750 17512000 >2000

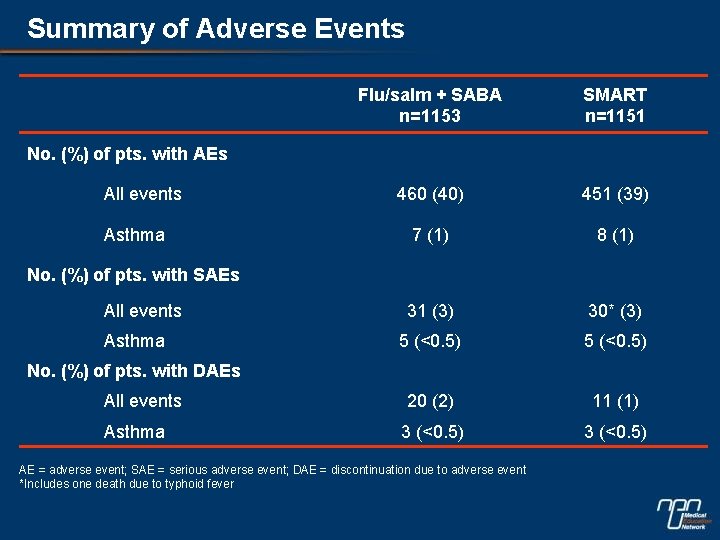
Summary of Adverse Events Flu/salm + SABA n=1153 SMART n=1151 460 (40) 451 (39) 7 (1) 8 (1) 31 (3) 30* (3) 5 (<0. 5) All events 20 (2) 11 (1) Asthma 3 (<0. 5) No. (%) of pts. with AEs All events Asthma No. (%) of pts. with SAEs All events Asthma No. (%) of pts. with DAEs AE = adverse event; SAE = serious adverse event; DAE = discontinuation due to adverse event *Includes one death due to typhoid fever

The COSMOS Study

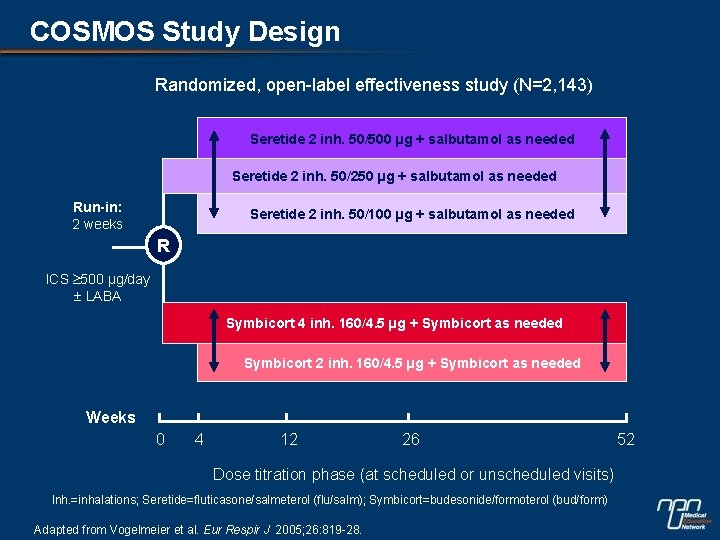
COSMOS Study Design Randomized, open-label effectiveness study (N=2, 143) Seretide 2 inh. 50/500 µg + salbutamol as needed Seretide 2 inh. 50/250 µg + salbutamol as needed Run-in: 2 weeks Seretide 2 inh. 50/100 µg + salbutamol as needed R ICS 500 µg/day LABA Symbicort 4 inh. 160/4. 5 µg + Symbicort as needed Symbicort 2 inh. 160/4. 5 µg + Symbicort as needed Weeks 0 4 12 26 Dose titration phase (at scheduled or unscheduled visits) Inh. =inhalations; Seretide=fluticasone/salmeterol (flu/salm); Symbicort=budesonide/formoterol (bud/form) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 52

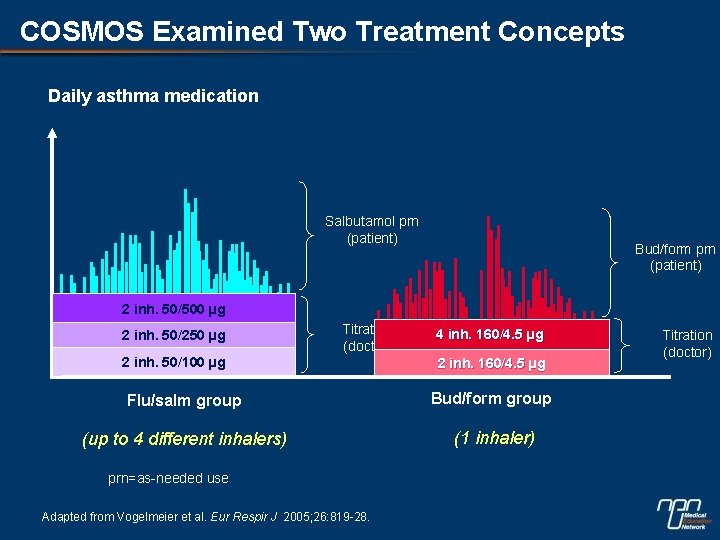
COSMOS Examined Two Treatment Concepts Daily asthma medication Salbutamol prn (patient) Bud/form prn (patient) 2 inh. 50/500 µg 2 inh. 50/250 µg Titration (doctor) 2 inh. 50/100 µg 4 inh. 160/4. 5 µg 2 inh. 160/4. 5 µg Flu/salm group Bud/form group (up to 4 different inhalers) (1 inhaler) prn=as-needed use Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. Titration (doctor)

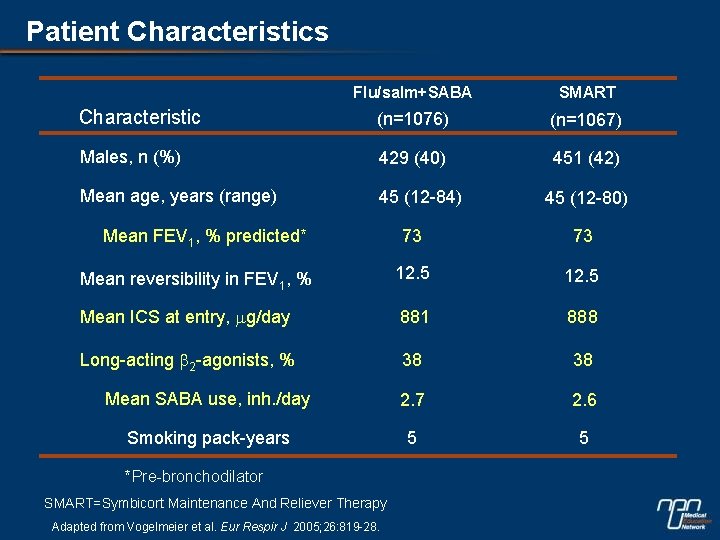
Patient Characteristics Flu/salm+SABA SMART Characteristic (n=1076) (n=1067) Males, n (%) 429 (40) 451 (42) Mean age, years (range) 45 (12 -84) Mean FEV 1, % predicted* 45 (12 -80) 73 73 Mean reversibility in FEV 1, % 12. 5 Mean ICS at entry, mg/day 881 888 Long-acting b 2 -agonists, % 38 38 Mean SABA use, inh. /day 2. 7 2. 6 Smoking pack-years 5 5 *Pre-bronchodilator SMART=Symbicort Maintenance And Reliever Therapy Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28.

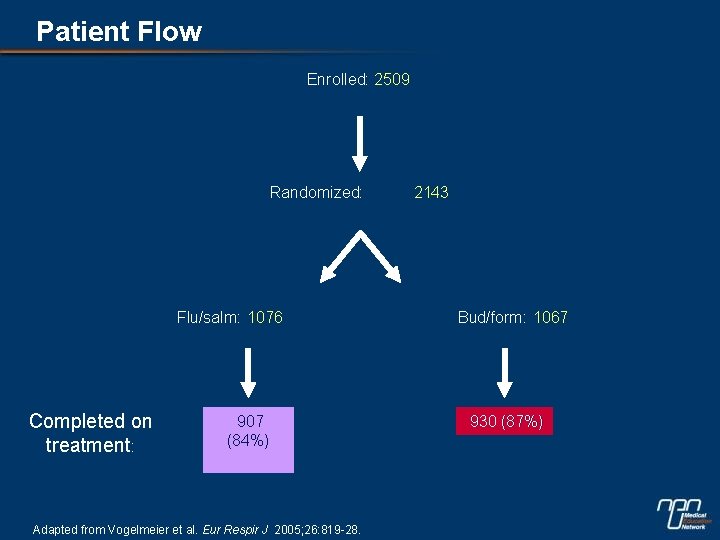
Patient Flow Enrolled: 2509 Randomized: Flu/salm: 1076 Completed on treatment: 907 (84%) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 2143 Bud/form: 1067 930 (87%)

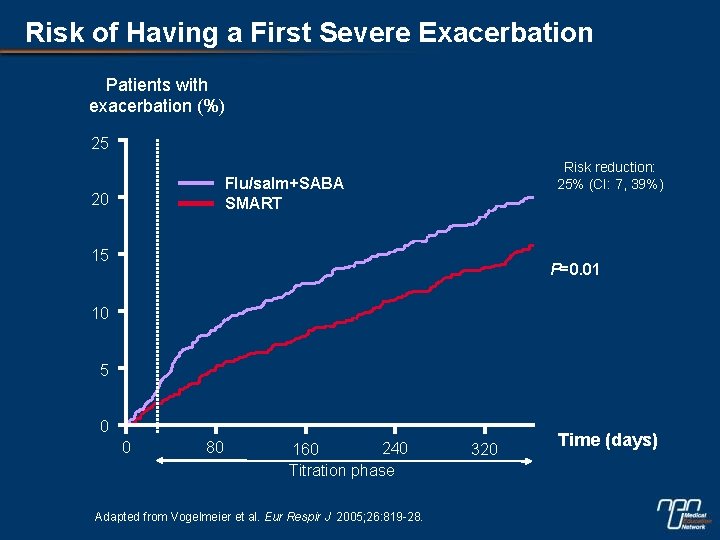
Risk of Having a First Severe Exacerbation Patients with exacerbation (%) 25 Risk reduction: 25% (CI: 7, 39%) Flu/salm+SABA SMART 20 15 P=0. 01 10 5 0 0 80 240 160 Titration phase Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 320 Time (days)

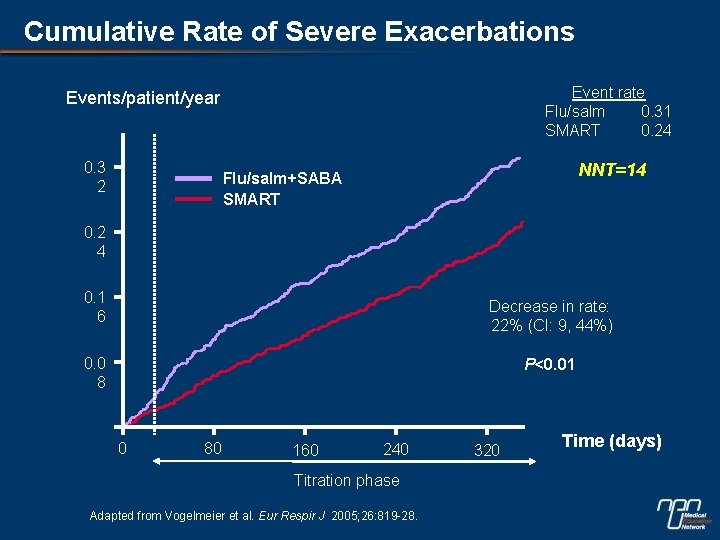
Cumulative Rate of Severe Exacerbations Event rate Flu/salm 0. 31 SMART 0. 24 Events/patient/year 0. 3 2 NNT=14 Flu/salm+SABA SMART 0. 2 4 0. 1 6 Decrease in rate: 22% (CI: 9, 44%) 0. 0 8 P<0. 01 0 80 160 240 Titration phase Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 320 Time (days)

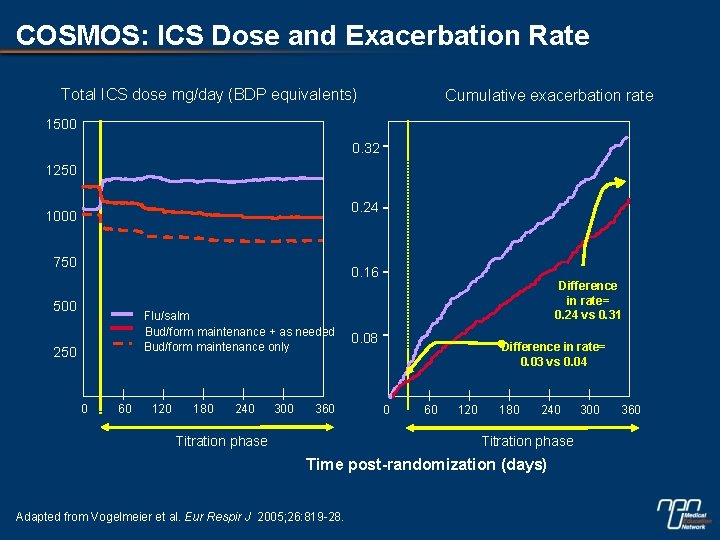
COSMOS: ICS Dose and Exacerbation Rate Total ICS dose mg/day (BDP equivalents) Cumulative exacerbation rate 1500 0. 32 1250 0. 24 1000 750 0. 16 500 Flu/salm Bud/form maintenance + as needed Bud/form maintenance only 250 0 60 120 180 240 300 360 Titration phase Difference in rate= 0. 24 vs 0. 31 0. 08 Difference in rate= 0. 03 vs 0. 04 0 60 120 180 240 Titration phase Time post-randomization (days) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 300 360

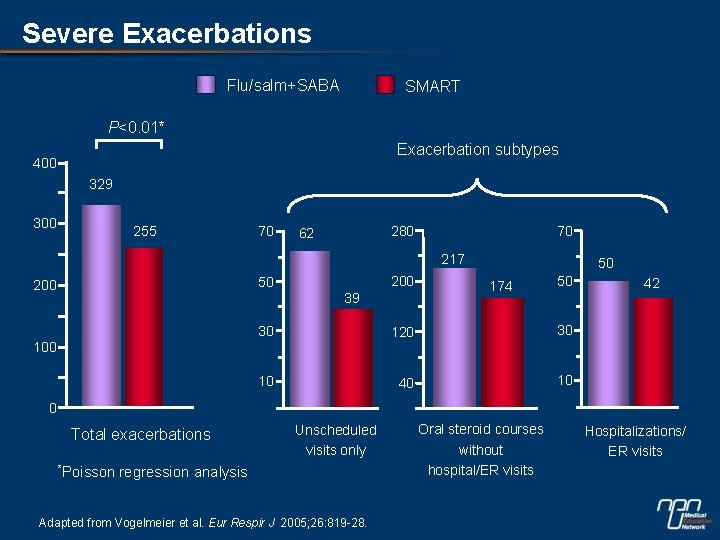
Severe Exacerbations Flu/salm+SABA SMART P<0. 01* Exacerbation subtypes 400 329 300 255 70 280 62 70 217 200 50 200 39 50 174 50 30 120 30 10 40 10 100 42 0 Total exacerbations *Poisson Unscheduled visits only regression analysis Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. Oral steroid courses without hospital/ER visits Hospitalizations/ ER visits

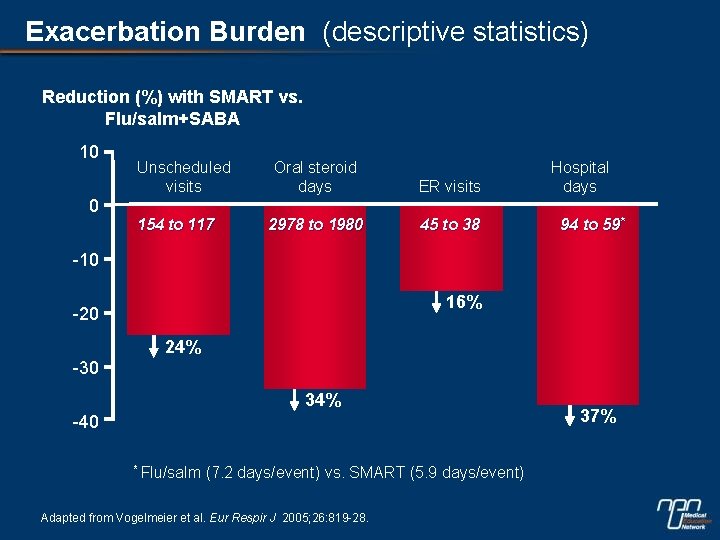
Exacerbation Burden (descriptive statistics) Reduction (%) with SMART vs. Flu/salm+SABA 10 Unscheduled visits Oral steroid days ER visits 2978 to 1980 45 to 38 Hospital days 0 154 to 117 94 to 59* -10 16% -20 24% -30 34% -40 * Flu/salm (7. 2 days/event) vs. SMART (5. 9 days/event) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 37%

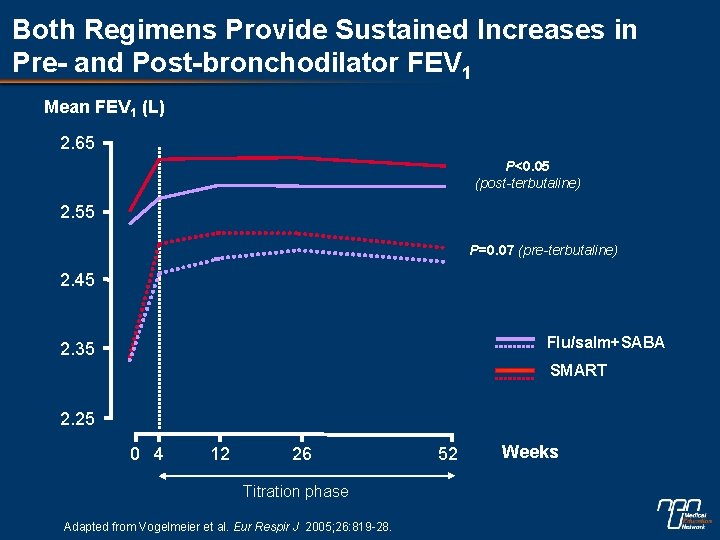
Both Regimens Provide Sustained Increases in Pre- and Post-bronchodilator FEV 1 Mean FEV 1 (L) 2. 65 P<0. 05 (post-terbutaline) 2. 55 P=0. 07 (pre-terbutaline) 2. 45 Flu/salm+SABA 2. 35 SMART 2. 25 0 4 12 26 Titration phase Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 52 Weeks

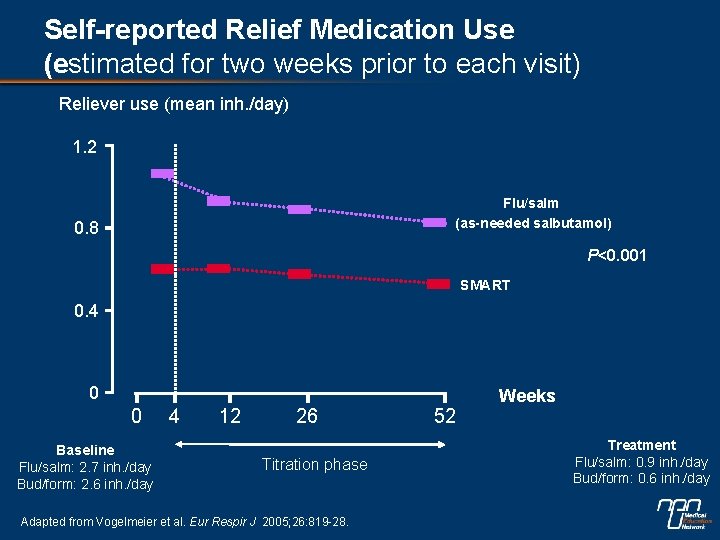
Self-reported Relief Medication Use (estimated for two weeks prior to each visit) Reliever use (mean inh. /day) 1. 2 Flu/salm (as-needed salbutamol) 0. 8 P<0. 001 SMART 0. 4 0 0 Baseline Flu/salm: 2. 7 inh. /day Bud/form: 2. 6 inh. /day 4 12 26 Titration phase Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 52 Weeks Treatment Flu/salm: 0. 9 inh. /day Bud/form: 0. 6 inh. /day

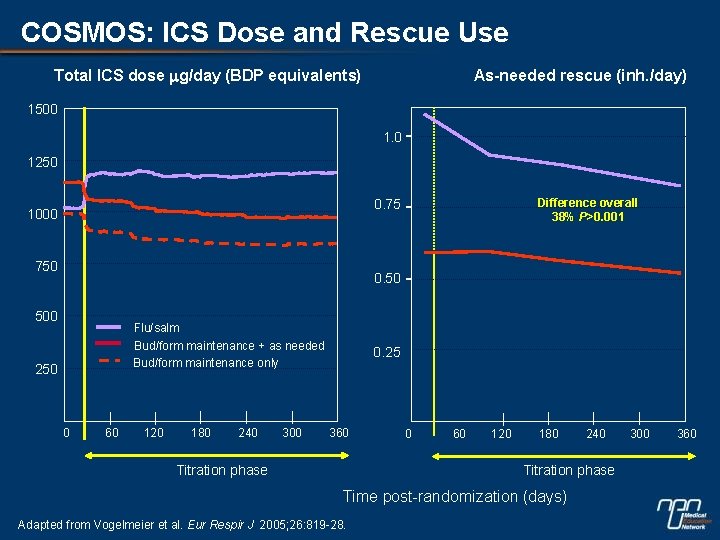
COSMOS: ICS Dose and Rescue Use Total ICS dose mg/day (BDP equivalents) As-needed rescue (inh. /day) 1500 1. 0 1250 Difference overall 38% P>0. 001 0. 75 1000 750 0. 50 500 Flu/salm Bud/form maintenance + as needed Bud/form maintenance only 250 0 60 120 180 240 300 0. 25 360 Titration phase 0 60 120 180 240 Titration phase Time post-randomization (days) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 300 360

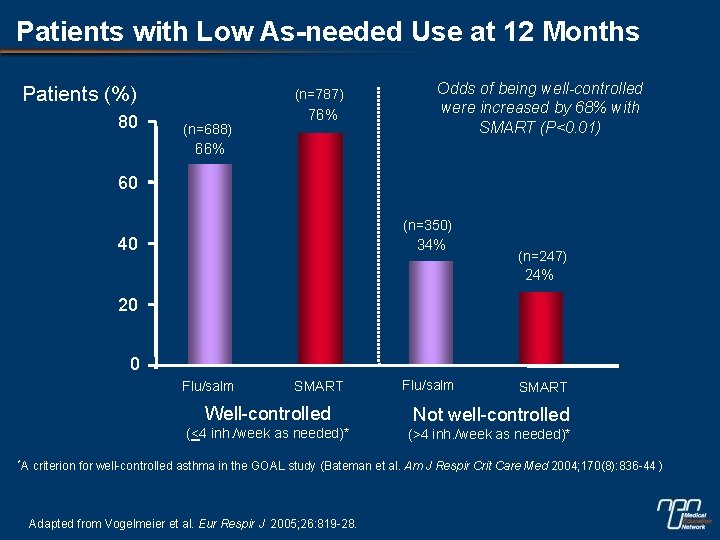
Patients with Low As-needed Use at 12 Months Patients (%) 80 (n=787) (n=688) 76% Odds of being well-controlled were increased by 68% with SMART (P<0. 01) 66% 60 (n=350) 40 34% (n=247) 24% 20 0 Flu/salm *A SMART Flu/salm SMART Well-controlled Not well-controlled (<4 inh. /week as needed)* (>4 inh. /week as needed)* criterion for well-controlled asthma in the GOAL study (Bateman et al. Am J Respir Crit Care Med 2004; 170(8): 836 -44 ) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28.

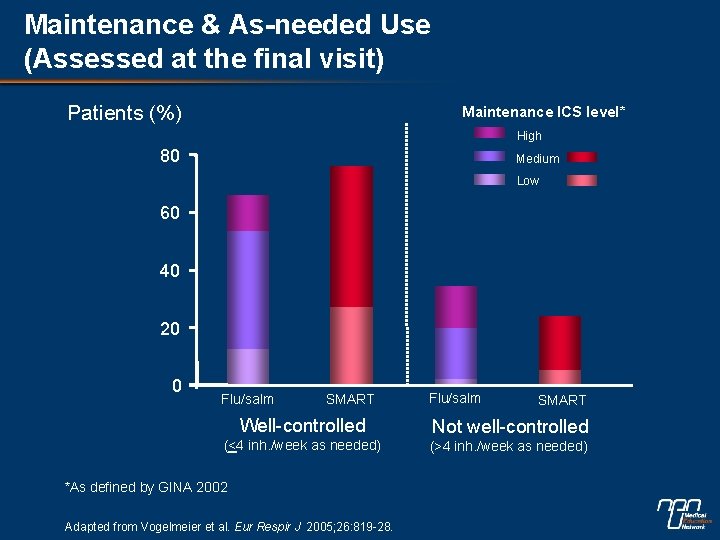
Maintenance & As-needed Use (Assessed at the final visit) Patients (%) Maintenance ICS level* High 80 Medium Low 60 40 20 0 Flu/salm SMART Well-controlled Not well-controlled (<4 inh. /week as needed) (>4 inh. /week as needed) *As defined by GINA 2002 Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28.

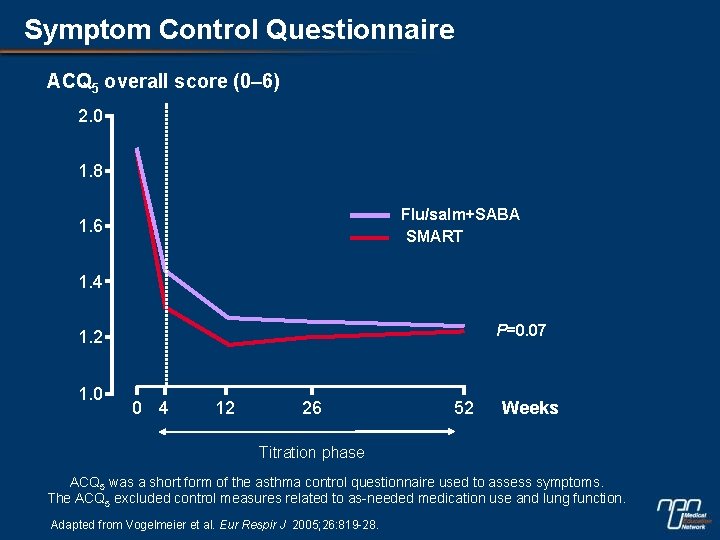
Symptom Control Questionnaire ACQ 5 overall score (0– 6) 2. 0 1. 8 Flu/salm+SABA SMART 1. 6 1. 4 P=0. 07 1. 2 1. 0 0 4 12 26 52 Weeks Titration phase ACQ 5 was a short form of the asthma control questionnaire used to assess symptoms. The ACQ 5 excluded control measures related to as-needed medication use and lung function. Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28.

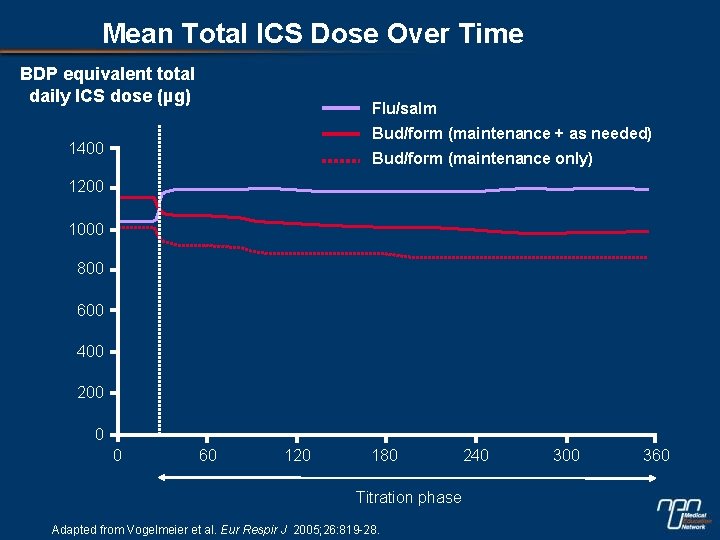
Mean Total ICS Dose Over Time BDP equivalent total daily ICS dose (µg) Flu/salm Bud/form (maintenance + as needed) 1400 Bud/form (maintenance only) 1200 1000 800 600 400 200 0 0 60 120 180 Titration phase Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. 240 300 360

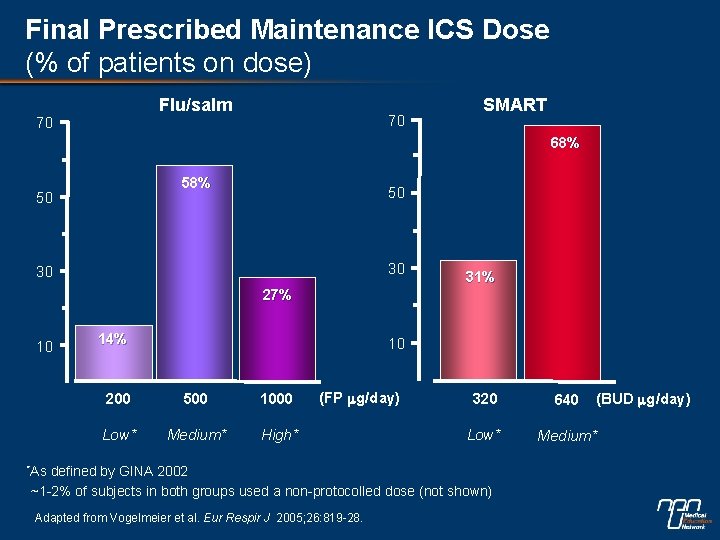
Final Prescribed Maintenance ICS Dose (% of patients on dose) Flu/salm 70 70 SMART 68% 50 50 30 30 31% 27% 10 14% 10 200 500 1000 Low* Medium* High* (FP mg/day) *As 640 Low* Medium* defined by GINA 2002 ~1 -2% of subjects in both groups used a non-protocolled dose (not shown) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. (BUD mg/day) 320

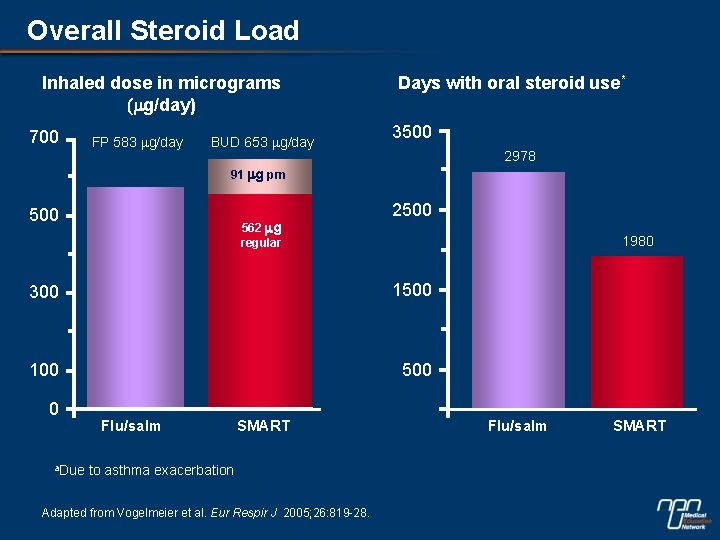
Overall Steroid Load Inhaled dose in micrograms (mg/day) 700 FP 583 mg/day BUD 653 mg/day Days with oral steroid use* 3500 2978 91 mg prn 500 562 mg regular 2500 1980 300 1500 100 500 0 a. Due Flu/salm SMART to asthma exacerbation Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. Flu/salm SMART

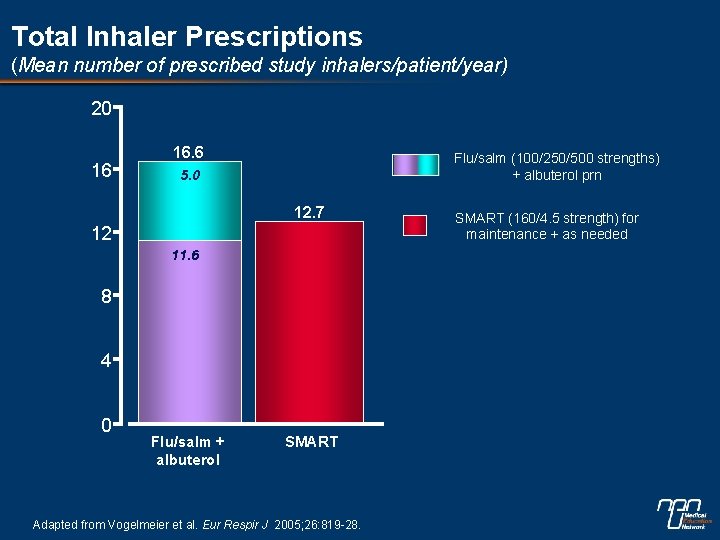
Total Inhaler Prescriptions (Mean number of prescribed study inhalers/patient/year) 20 16 16. 6 Flu/salm (100/250/500 strengths) + albuterol prn 5. 0 12. 7 12 11. 6 8 4 0 Flu/salm + albuterol SMART Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. SMART (160/4. 5 strength) for maintenance + as needed

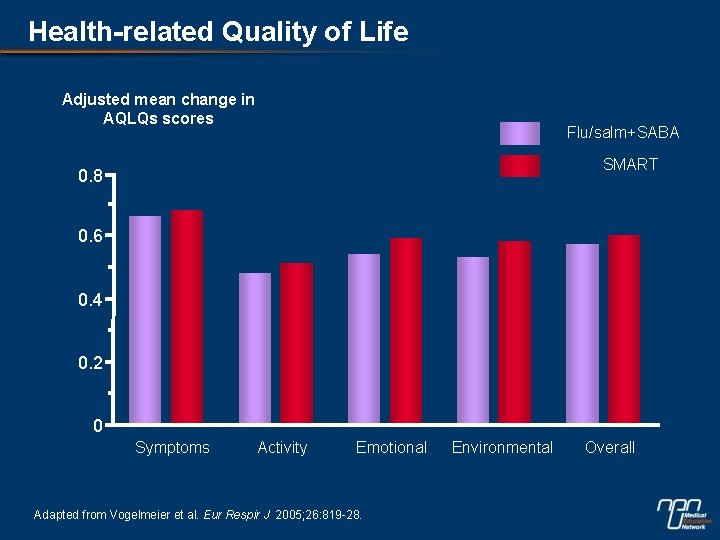
Health-related Quality of Life Adjusted mean change in AQLQs scores Flu/salm+SABA SMART 0. 8 0. 6 0. 4 0. 2 0 Symptoms Activity Emotional Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. Environmental Overall

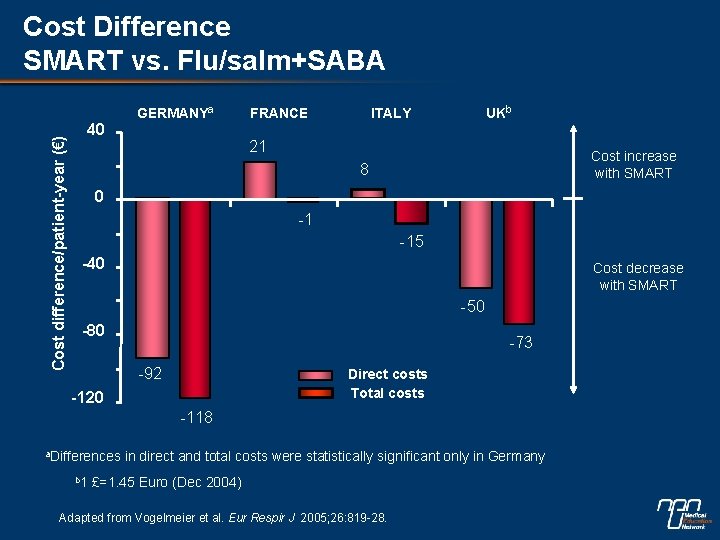
Cost difference/patient-year (€) Cost Difference SMART vs. Flu/salm+SABA 40 GERMANYa FRANCE UKb ITALY 21 Cost increase with SMART 8 0 -1 -15 -40 Cost decrease with SMART -50 -80 -73 -92 Direct costs Total costs -120 -118 a. Differences b 1 in direct and total costs were statistically significant only in Germany £=1. 45 Euro (Dec 2004) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28.

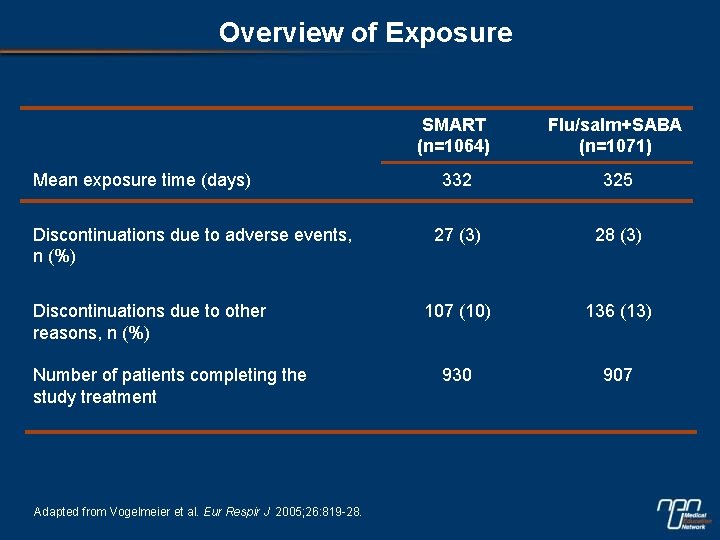
Overview of Exposure Mean exposure time (days) Discontinuations due to adverse events, n (%) Discontinuations due to other reasons, n (%) Number of patients completing the study treatment Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. SMART (n=1064) Flu/salm+SABA (n=1071) 332 325 27 (3) 28 (3) 107 (10) 136 (13) 930 907

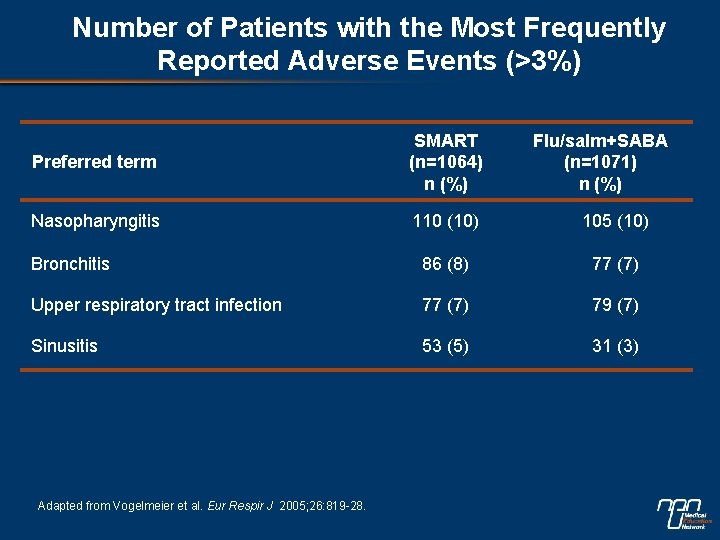
Number of Patients with the Most Frequently Reported Adverse Events (>3%) Preferred term SMART (n=1064) n (%) Nasopharyngitis 110 (10) 105 (10) Bronchitis 86 (8) 77 (7) Upper respiratory tract infection 77 (7) 79 (7) Sinusitis 53 (5) 31 (3) Adapted from Vogelmeier et al. Eur Respir J 2005; 26: 819 -28. Flu/salm+SABA (n=1071) n (%)

The COMPASS Study

Study Objective · To compare the efficacy and safety of budesonide/formoterol (bud/form) maintenance and reliever therapy (SMART) with: · Double maintenance dose of bud/form · Fluticasone/salmeterol (flu/salm) at its most frequently prescribed dose SMART = Symbicort Maintenance And Reliever Therapy Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36.

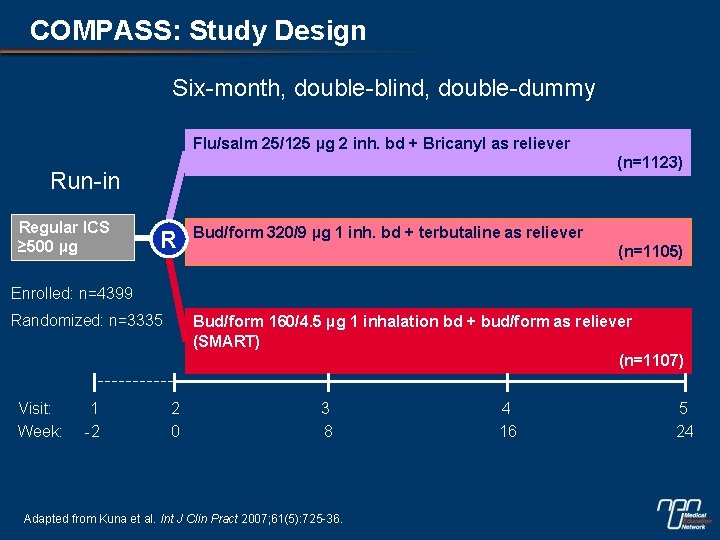
COMPASS: Study Design Six-month, double-blind, double-dummy Flu/salm 25/125 µg 2 inh. bd + Bricanyl as reliever (n=1123) Run-in Regular ICS ≥ 500 µg R Bud/form 320/9 µg 1 inh. bd + terbutaline as reliever (n=1105) Enrolled: n=4399 Randomized: n=3335 Visit: Week: 1 -2 Bud/form 160/4. 5 µg 1 inhalation bd + bud/form as reliever (SMART) (n=1107) 2 0 3 8 Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. 4 16 5 24

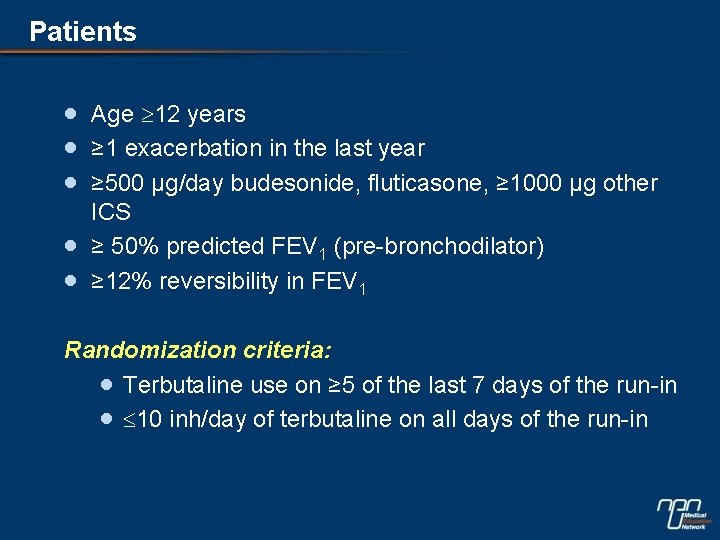
Patients · · · Age 12 years ≥ 1 exacerbation in the last year ≥ 500 µg/day budesonide, fluticasone, ≥ 1000 µg other ICS ≥ 50% predicted FEV 1 (pre-bronchodilator) ≥ 12% reversibility in FEV 1 Randomization criteria: · Terbutaline use on ≥ 5 of the last 7 days of the run-in · 10 inh/day of terbutaline on all days of the run-in

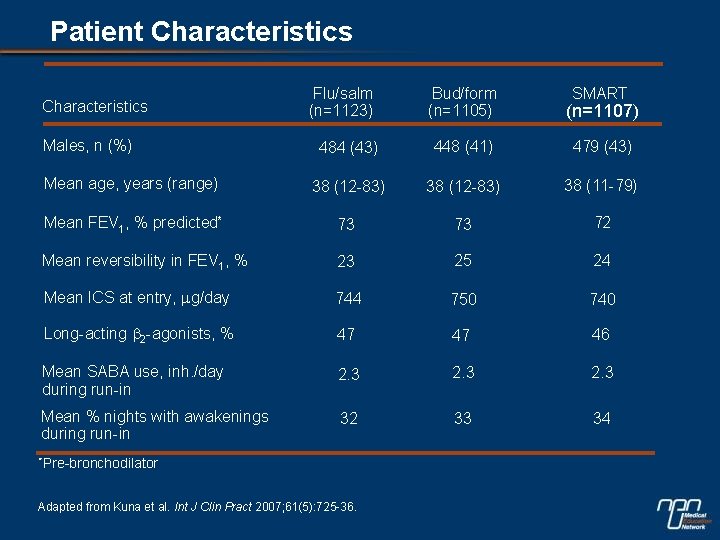
Patient Characteristics Flu/salm (n=1123) Bud/form (n=1105) (n=1107) 484 (43) 448 (41) 479 (43) Mean age, years (range) 38 (12 -83) 38 (11 -79) Mean FEV 1, % predicted* 73 73 72 Mean reversibility in FEV 1, % 23 25 24 Mean ICS at entry, mg/day 744 750 740 Long-acting b 2 -agonists, % 47 47 46 Mean SABA use, inh. /day during run-in 2. 3 Mean % nights with awakenings during run-in 32 33 34 Characteristics Males, n (%) *Pre-bronchodilator Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. SMART

Severe Exacerbations A deterioration in asthma leading to: · Emergency room treatment/hospitalization · Oral steroids for 3 days Primary variable: time to first severe exacerbation Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36.

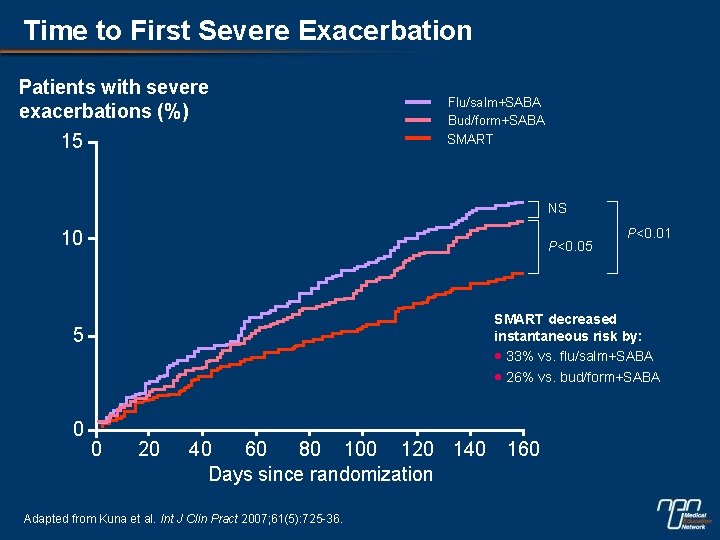
Time to First Severe Exacerbation Patients with severe exacerbations (%) 15 Flu/salm+SABA Bud/form+SABA SMART NS 10 P<0. 05 SMART decreased instantaneous risk by: · 33% vs. flu/salm+SABA · 26% vs. bud/form+SABA 5 0 P<0. 01 0 20 40 60 80 100 120 140 Days since randomization Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. 160

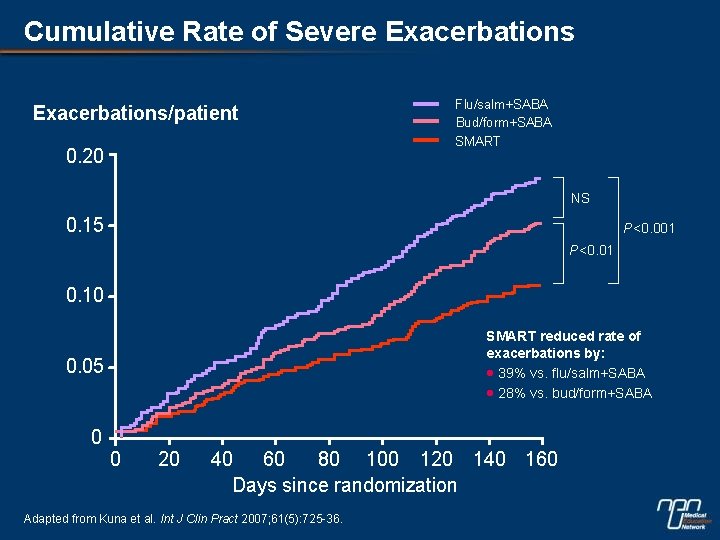
Cumulative Rate of Severe Exacerbations/patient 0. 20 Flu/salm+SABA Bud/form+SABA SMART NS 0. 15 P<0. 001 P<0. 01 0. 10 SMART reduced rate of exacerbations by: · 39% vs. flu/salm+SABA · 28% vs. bud/form+SABA 0. 05 0 0 20 40 60 80 100 120 140 160 Days since randomization Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36.

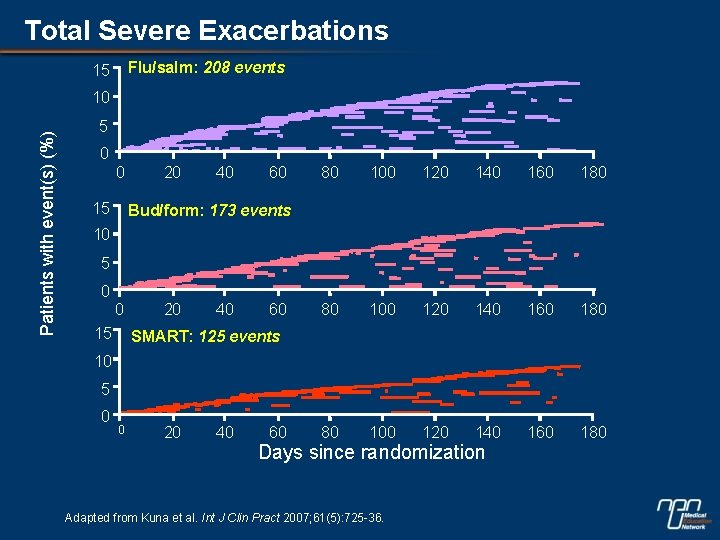
Total Severe Exacerbations Flu/salm: 208 events 15 Patients with event(s) (%) 10 5 0 0 15 20 40 60 80 100 120 140 160 180 Bud/form: 173 events 10 5 0 0 15 20 40 60 SMART: 125 events 10 5 0 0 20 40 60 Days since randomization Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36.

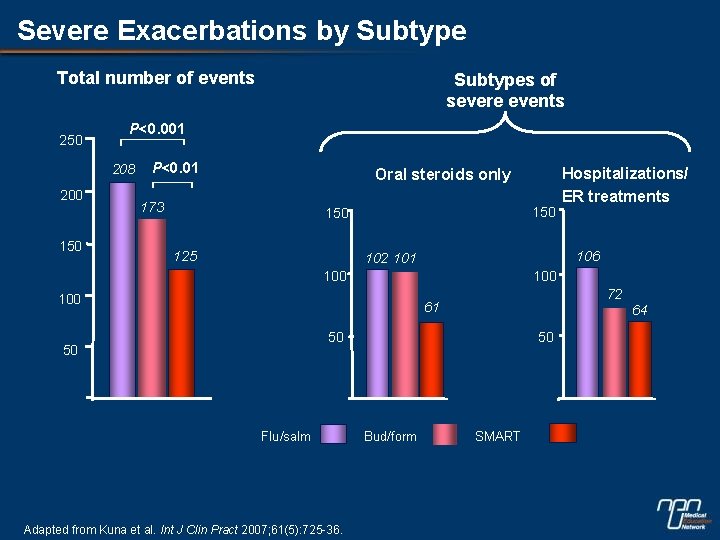
Severe Exacerbations by Subtype Total number of events 250 P<0. 001 208 200 150 Subtypes of severe events P<0. 01 Oral steroids only 173 150 125 106 102 101 100 100 72 61 50 50 50 Flu/salm Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. Hospitalizations/ ER treatments Bud/form SMART 64

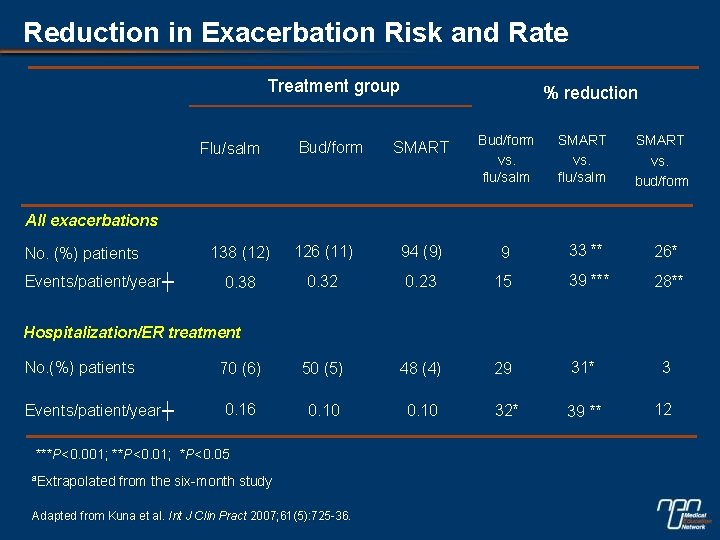
Reduction in Exacerbation Risk and Rate Treatment group Flu/salm Bud/form % reduction SMART Bud/form vs. flu/salm SMART vs. bud/form All exacerbations No. (%) patients Events/patient/year┼ 138 (12) 126 (11) 94 (9) 9 33 ** 26* 0. 38 0. 32 0. 23 15 39 *** 28** 70 (6) 50 (5) 48 (4) 29 31* 3 0. 16 0. 10 32* 39 ** 12 Hospitalization/ER treatment No. (%) patients Events/patient/year┼ ***P<0. 001; **P<0. 01; *P<0. 05 ªExtrapolated from the six-month study Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36.

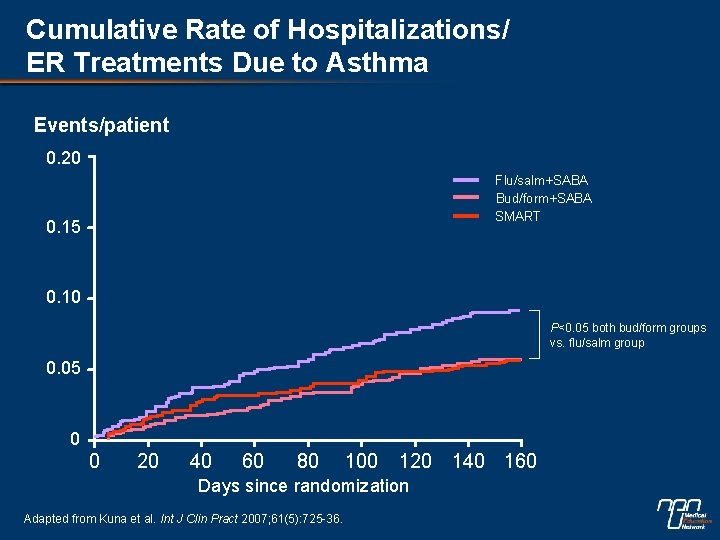
Cumulative Rate of Hospitalizations/ ER Treatments Due to Asthma Events/patient 0. 20 Flu/salm+SABA Bud/form+SABA SMART 0. 15 0. 10 P<0. 05 both bud/form groups vs. flu/salm group 0. 05 0 0 20 40 60 80 100 120 140 160 Days since randomization Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36.

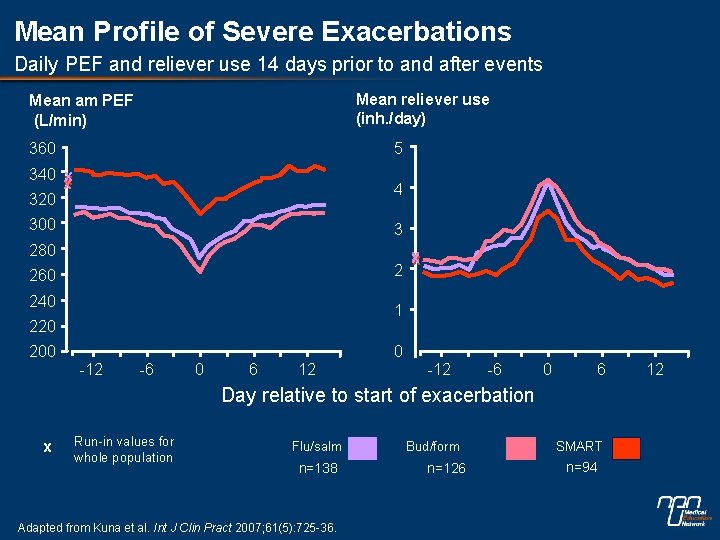
Mean Profile of Severe Exacerbations Daily PEF and reliever use 14 days prior to and after events Mean reliever use (inh. /day) Mean am PEF (L/min) 360 5 340 x x x 320 4 300 3 280 2 260 240 x 1 220 200 0 -12 -6 0 6 Day relative to start of exacerbation x Run-in values for whole population Flu/salm n=138 Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. Bud/form n=126 SMART n=94 12

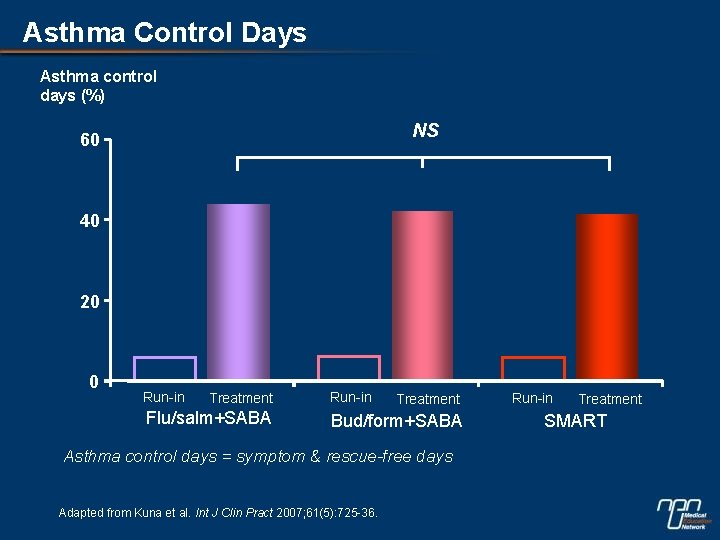
Asthma Control Days Asthma control days (%) NS 60 40 20 0 Run-in Treatment Flu/salm+SABA Run-in Treatment Bud/form+SABA Asthma control days = symptom & rescue-free days Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. Run-in Treatment SMART

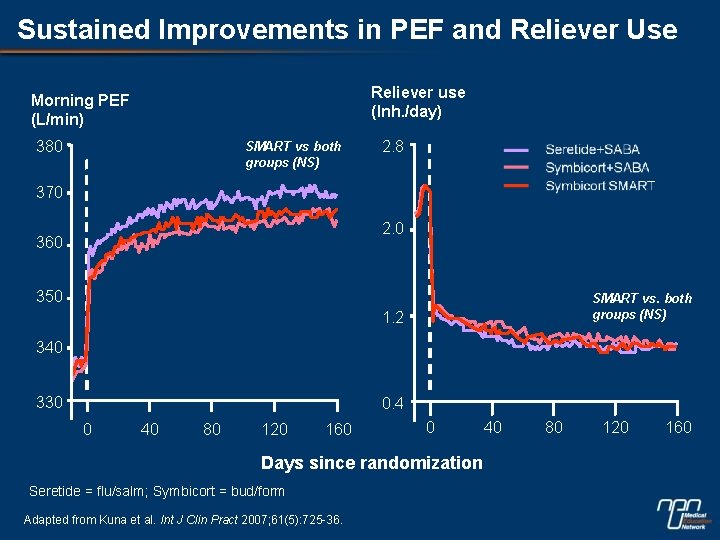
Sustained Improvements in PEF and Reliever Use Reliever use (Inh. /day) Morning PEF (L/min) 380 SMART vs both groups (NS) 2. 8 370 2. 0 360 350 SMART vs. both groups (NS) 1. 2 340 330 0. 4 0 40 80 120 160 0 Days since randomization Seretide = flu/salm; Symbicort = bud/form Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. 40 80 120 160

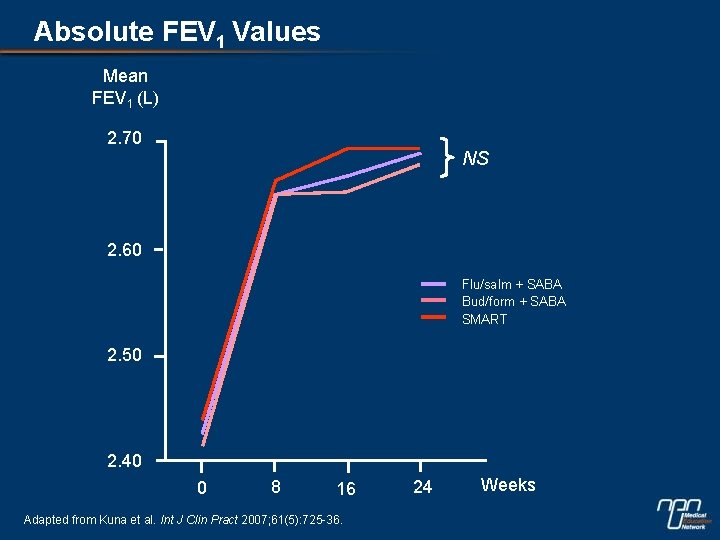
Absolute FEV 1 Values Mean FEV 1 (L) 2. 70 NS 2. 60 Flu/salm + SABA Bud/form + SABA SMART 2. 50 2. 40 0 8 16 Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. 24 Weeks

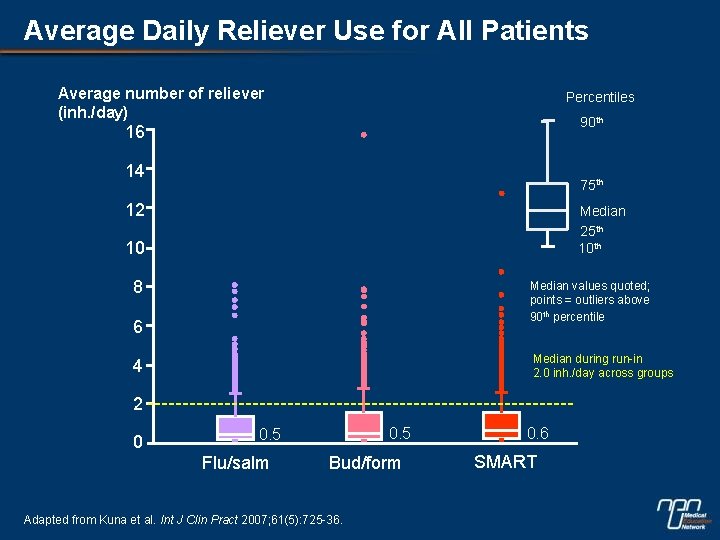
Average Daily Reliever Use for All Patients Average number of reliever (inh. /day) Percentiles 90 th 16 14 75 th 12 Median 25 th 10 8 Median values quoted; points = outliers above 90 th percentile 6 Median during run-in 2. 0 inh. /day across groups 4 2 0 0. 5 Flu/salm Bud/form Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36. 0. 6 SMART

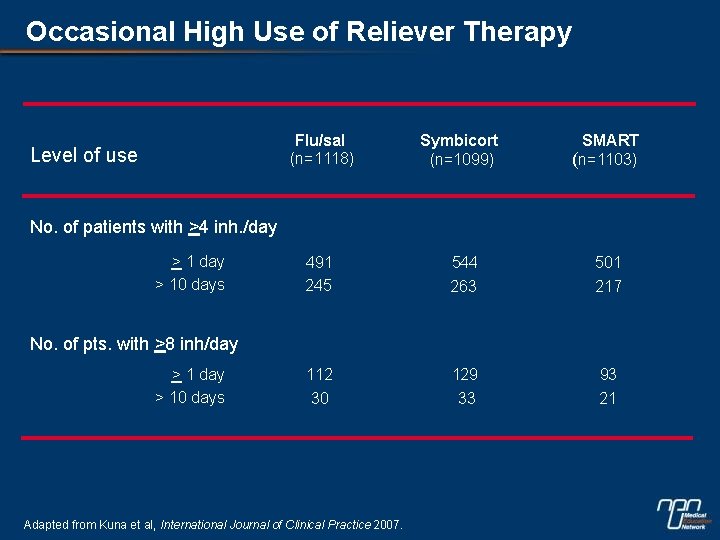
Occasional High Use of Reliever Therapy Level of use Flu/sal (n=1118) Symbicort (n=1099) SMART (n=1103) 491 245 544 263 501 217 112 30 129 33 93 21 No. of patients with >4 inh. /day > 10 days No. of pts. with >8 inh/day > 10 days Adapted from Kuna et al, International Journal of Clinical Practice 2007.

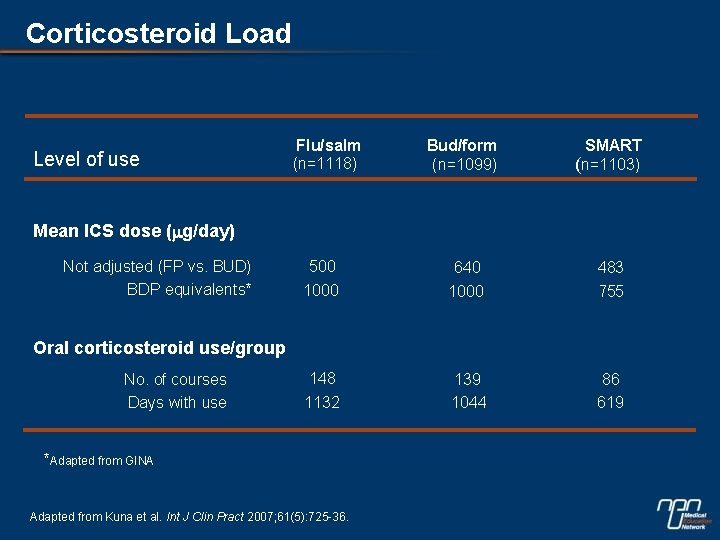
Corticosteroid Load Level of use Flu/salm (n=1118) Bud/form (n=1099) SMART (n=1103) 500 1000 640 1000 483 755 148 1132 139 1044 86 619 Mean ICS dose (mg/day) Not adjusted (FP vs. BUD) BDP equivalents* Oral corticosteroid use/group No. of courses Days with use *Adapted from GINA Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36.

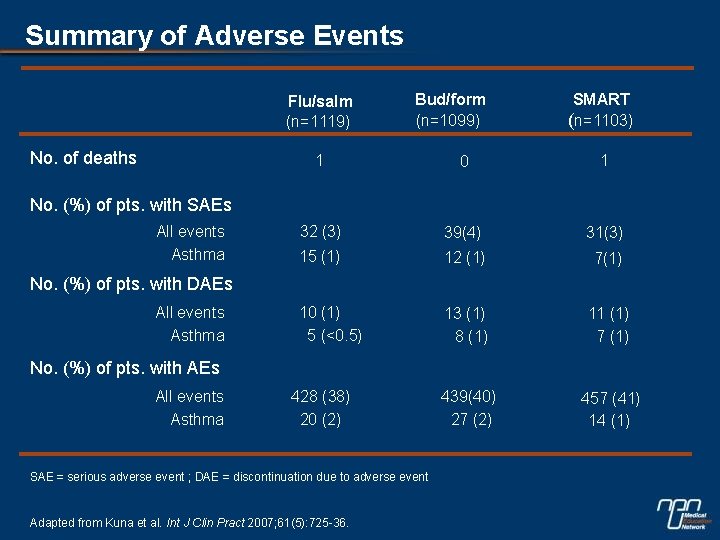
Summary of Adverse Events Flu/salm (n=1119) No. of deaths Bud/form (n=1099) SMART (n=1103) 1 0 1 32 (3) 39(4) 31(3) 15 (1) 12 (1) 7(1) 10 (1) 5 (<0. 5) 13 (1) 8 (1) 11 (1) 7 (1) 439(40) 27 (2) 457 (41) 14 (1) No. (%) of pts. with SAEs All events Asthma No. (%) of pts. with DAEs All events Asthma No. (%) of pts. with AEs All events Asthma 428 (38) 20 (2) SAE = serious adverse event ; DAE = discontinuation due to adverse event Adapted from Kuna et al. Int J Clin Pract 2007; 61(5): 725 -36.