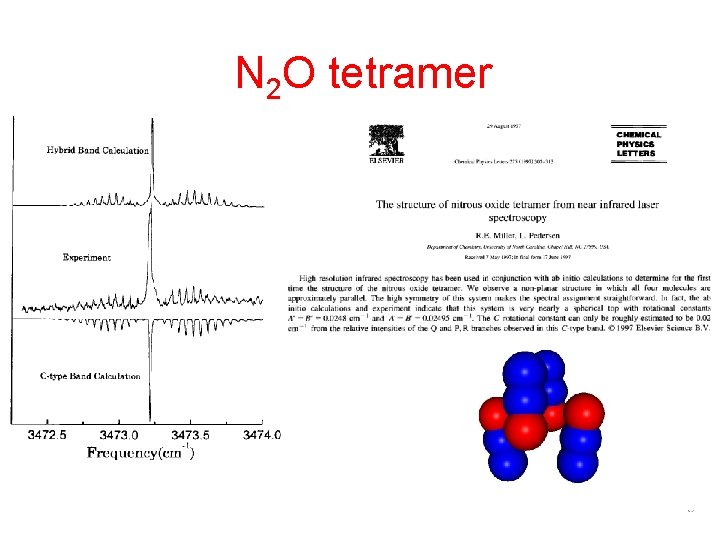
STRUCTURES OF TWO ISOMERS OF NITROUS OXIDE TETRAMER




- Slides: 18

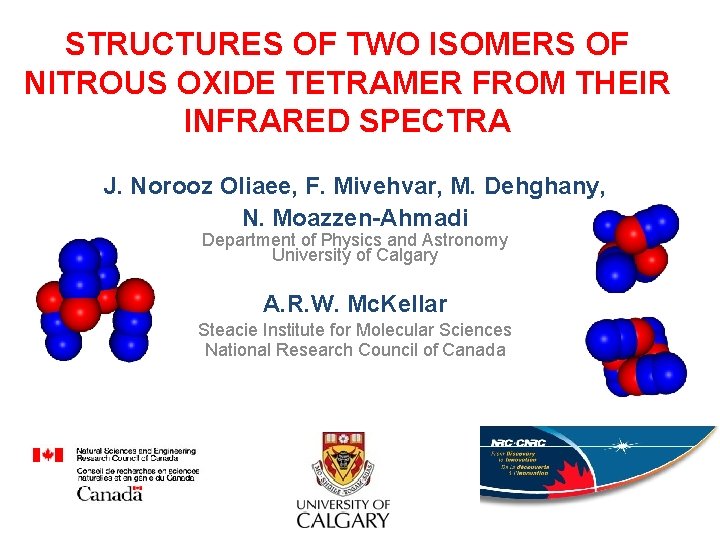
STRUCTURES OF TWO ISOMERS OF NITROUS OXIDE TETRAMER FROM THEIR INFRARED SPECTRA J. Norooz Oliaee, F. Mivehvar, M. Dehghany, N. Moazzen-Ahmadi Department of Physics and Astronomy University of Calgary A. R. W. Mc. Kellar Steacie Institute for Molecular Sciences National Research Council of Canada

Motivation • Weakly bound complexes provide a convenient starting point for a detailed understanding of different pathways that can be taken between the gas and condensed phases of matter. • It is of considerable interest to determine the number of isomers for a cluster size and if and how geometrical choices made in the early stages of condensation influence the growth of larger clusters. • Although it is expected that the number of isomers grows rapidly with cluster size, in many cases only a single isomer is observed experimentally.

Summary of past works on N 2 O clusters The N 2 O dimer was also observed in the regions of the 1 band (Qian, Herrebout, Howard, 1997) and the 3 band Non-polar dimer (Oshima, Matsumoto, Takami, Kuchitsu, 1988)

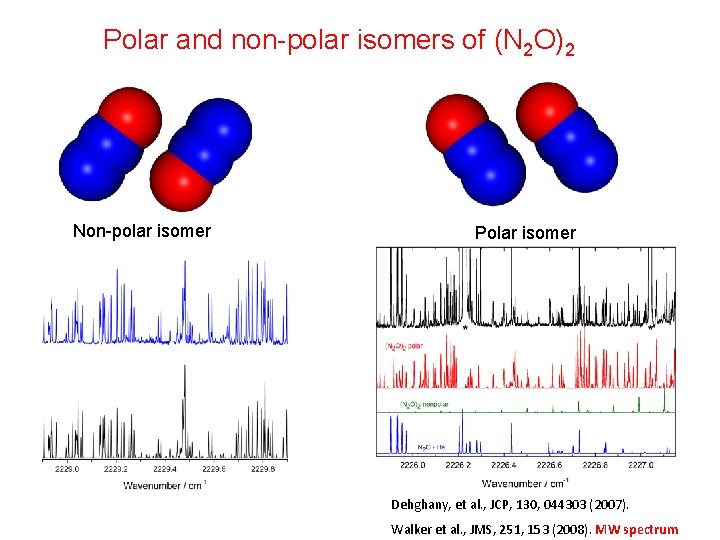
Polar and non-polar isomers of (N 2 O)2 Non-polar isomer Polar isomer Dehghany, et al. , JCP, 130, 044303 (2007). Walker et al. , JMS, 251, 153 (2008). MW spectrum

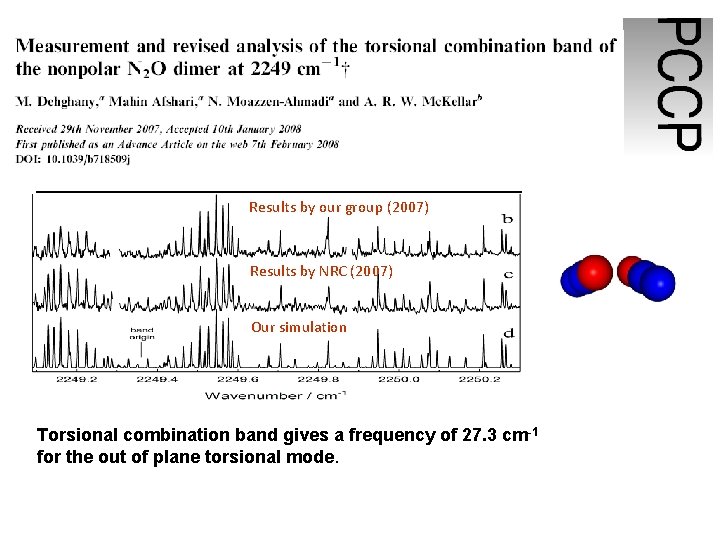
Simulation based on the results by Hecker et al. (2003) Results by our group (2007) Results by NRC (2007) Our simulation Torsional combination band gives a frequency of 27. 3 cm-1 for the out of plane torsional mode.

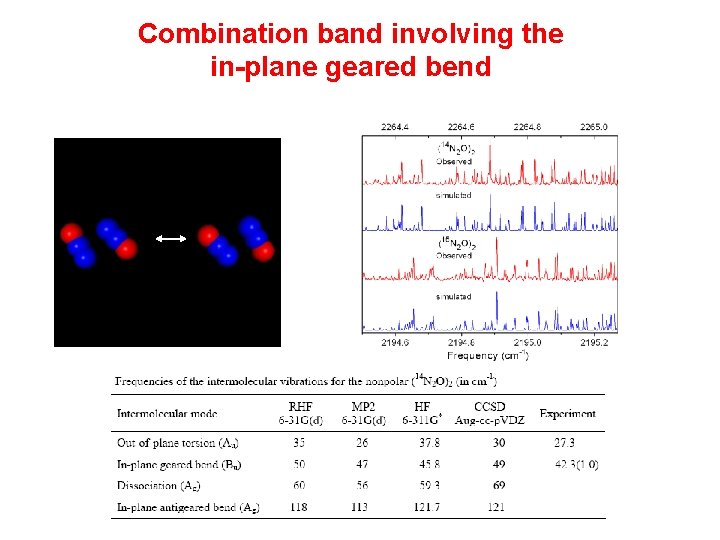
Combination band involving the in-plane geared bend

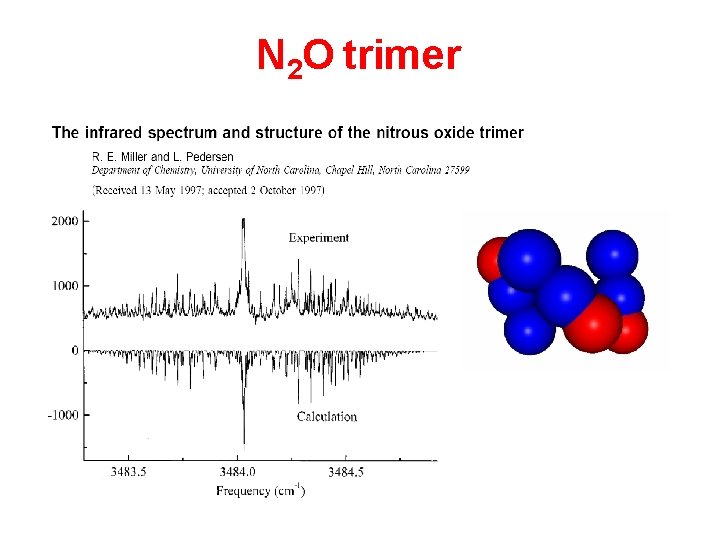
N 2 O trimer

The barrel-shaped N 2 O trimer (14 N 2 O)3 (15 N 2 O)3 Three bands for (14 N 2 O)3 in the 1 region of N 2 O monomer Two bands for (14 N 2 O)3 in the 3 region of N 2 O monomer (1280 cm-1) Analogous bands for (15 N 2 O)3

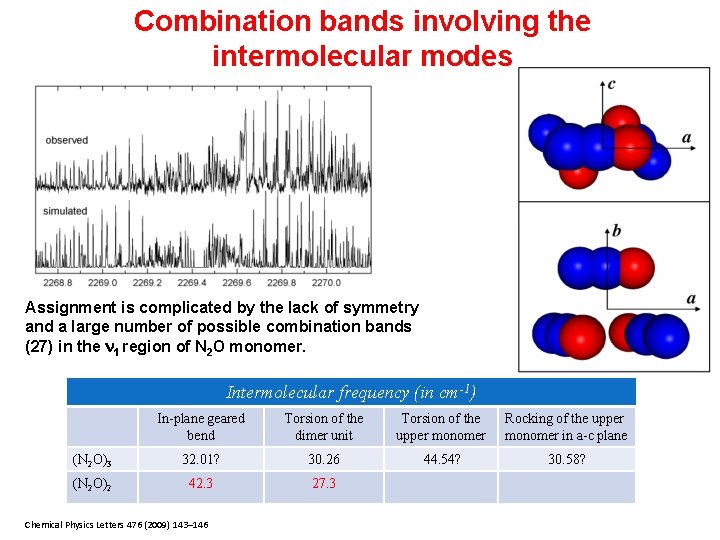
Combination bands involving the intermolecular modes Assignment is complicated by the lack of symmetry and a large number of possible combination bands (27) in the 1 region of N 2 O monomer. Intermolecular frequency (in cm-1) In-plane geared bend Torsion of the dimer unit Torsion of the upper monomer Rocking of the upper monomer in a-c plane (N 2 O)3 32. 01? 30. 26 44. 54? 30. 58? (N 2 O)2 42. 3 27. 3 Chemical Physics Letters 476 (2009) 143– 146

N 2 O tetramer

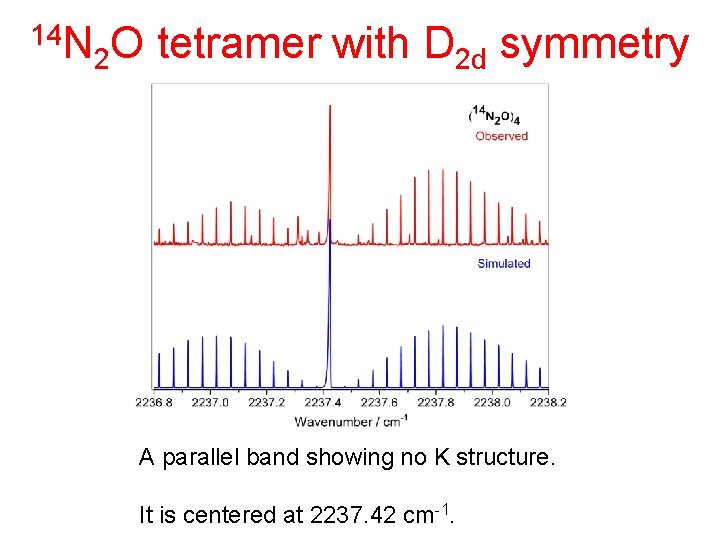
14 N 2 O tetramer with D 2 d symmetry A parallel band showing no K structure. It is centered at 2237. 42 cm-1.

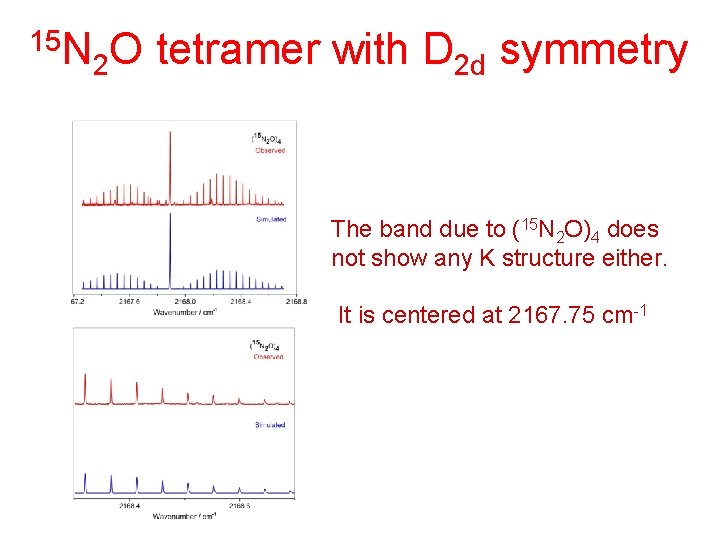
15 N 2 O tetramer with D 2 d symmetry The band due to (15 N 2 O)4 does not show any K structure either. It is centered at 2167. 75 cm-1

Mixed isotopomer (15 N 2 O)3(14 N 2 O) c-type band can be well simulated using Cs symmetry. A = 726. 73 MHz B = 719. 28 MHz C = 675. 99 MHz

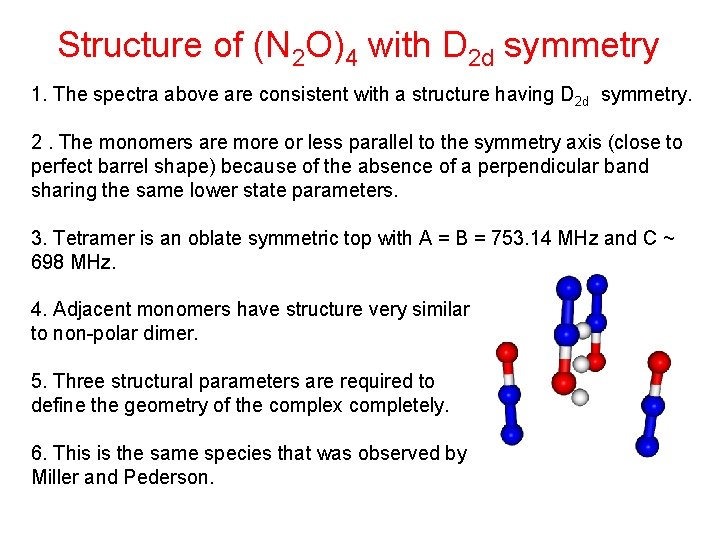
Structure of (N 2 O)4 with D 2 d symmetry 1. The spectra above are consistent with a structure having D 2 d symmetry. 2. The monomers are more or less parallel to the symmetry axis (close to perfect barrel shape) because of the absence of a perpendicular band sharing the same lower state parameters. 3. Tetramer is an oblate symmetric top with A = B = 753. 14 MHz and C ~ 698 MHz. 4. Adjacent monomers have structure very similar to non-polar dimer. 5. Three structural parameters are required to define the geometry of the complex completely. 6. This is the same species that was observed by Miller and Pederson.

A second N 2 O tetramer band: this one is perpendicular, and located close to the trimer band The 15 N 16 O isotopomer is shown here For the normal isotope, this band is centered at 2232. 209 cm-1

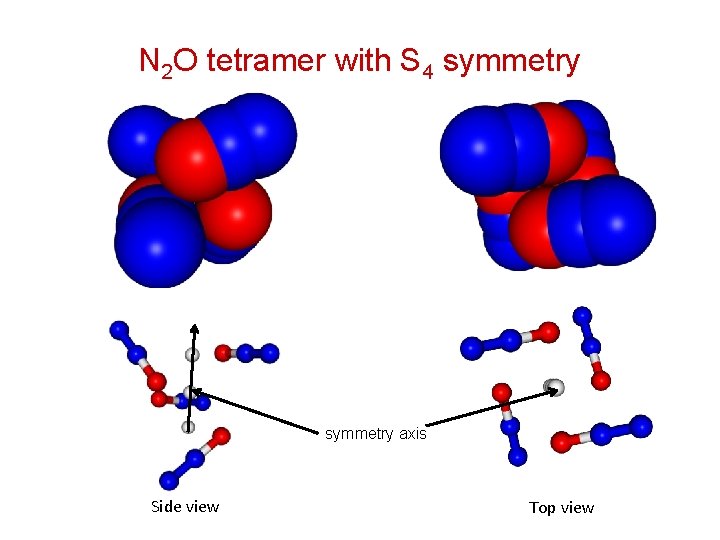
N 2 O tetramer with S 4 symmetry Side view symmetry axis Side view Top view

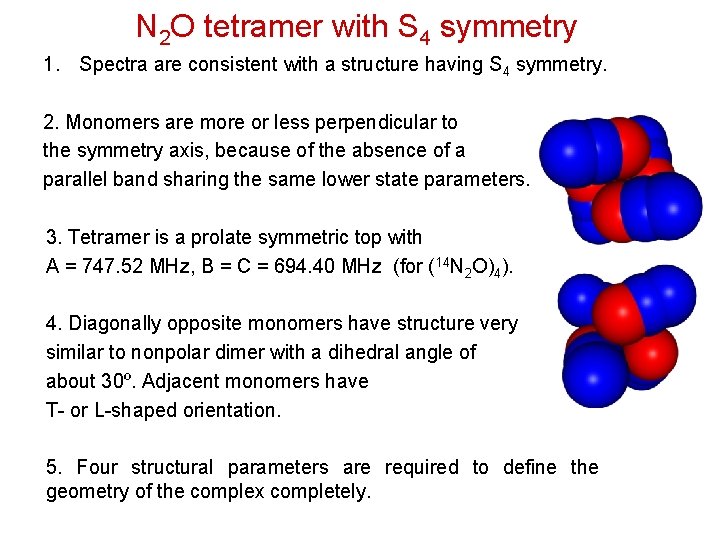
N 2 O tetramer with S 4 symmetry 1. Spectra are consistent with a structure having S 4 symmetry. 2. Monomers are more or less perpendicular to the symmetry axis, because of the absence of a parallel band sharing the same lower state parameters. 3. Tetramer is a prolate symmetric top with A = 747. 52 MHz, B = C = 694. 40 MHz (for (14 N 2 O)4). 4. Diagonally opposite monomers have structure very similar to nonpolar dimer with a dihedral angle of about 30º. Adjacent monomers have T- or L-shaped orientation. 5. Four structural parameters are required to define the geometry of the complex completely.

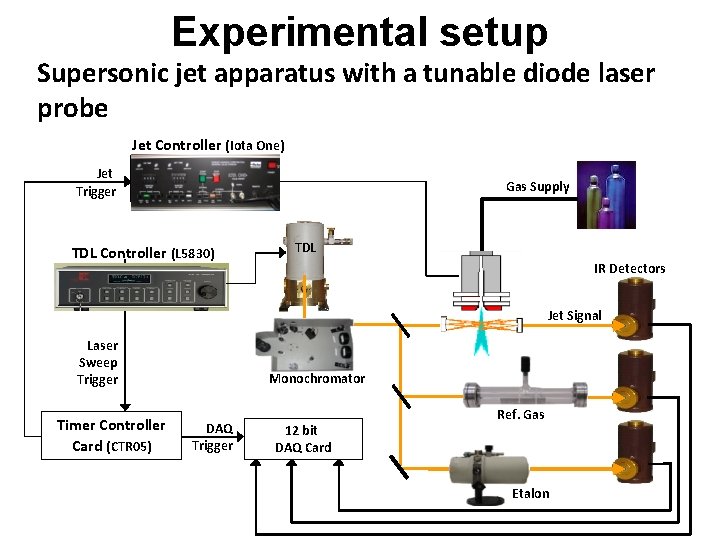
Experimental setup Supersonic jet apparatus with a tunable diode laser probe Jet Controller (Iota One) Jet Trigger Jet Controller TDL Controller (L 5830) Gas Supply TDL IR Detectors Jet Signal Laser Sweep Trigger Timer Controller Card (CTR 05) Monochromator DAQ Trigger 12 bit DAQ Card Ref. Gas Etalon
 Nitrous oxide cylinder uses
Nitrous oxide cylinder uses Depressan
Depressan Nitrous oxide
Nitrous oxide Inhalation anesthetics
Inhalation anesthetics Nitrous oxide
Nitrous oxide Most inhalants are actually intended to be
Most inhalants are actually intended to be Poynting effect nitrous oxide
Poynting effect nitrous oxide Enantiomer vs diastereomer
Enantiomer vs diastereomer Acidic oxide and basic oxide difference
Acidic oxide and basic oxide difference Cell biology cooper
Cell biology cooper Introduction of cytoskeleton
Introduction of cytoskeleton Hb tetramer
Hb tetramer Lac repressor tetramer
Lac repressor tetramer Perchloric acid and barium hydroxide
Perchloric acid and barium hydroxide Primary amine and nitrous acid
Primary amine and nitrous acid Asep saefumillah
Asep saefumillah Homology
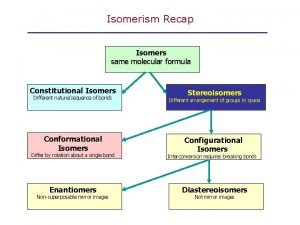
Homology Optical isomers of octahedral complexes
Optical isomers of octahedral complexes Nonsuperposable
Nonsuperposable