STRUCTURES Module C 2 Covalent Bonding Describe simply

STRUCTURES Module C 2
Covalent Bonding • Describe, simply, how atoms form covalent bonds (P) • List the properties of simple covalent compounds and giant covalent structures (P) • Construct dot and cross diagrams for single covalent bonds (hydrogen, chlorine, fluorine, water) (P) • Construct dot and cross diagrams for double covalent bonds (oxygen, carbon dioxide) (M) • Explain the properties of simple covalent compounds (M) • Construct dot and cross diagrams of complex covalent compounds including triple bonds (D) • Explain the properties of diamond and graphite (D)
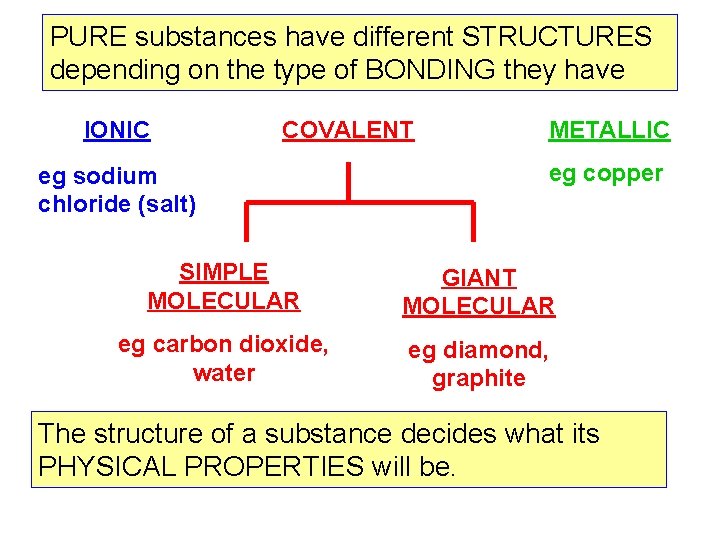
PURE substances have different STRUCTURES depending on the type of BONDING they have IONIC COVALENT eg sodium chloride (salt) METALLIC eg copper SIMPLE MOLECULAR GIANT MOLECULAR eg carbon dioxide, water eg diamond, graphite The structure of a substance decides what its PHYSICAL PROPERTIES will be.
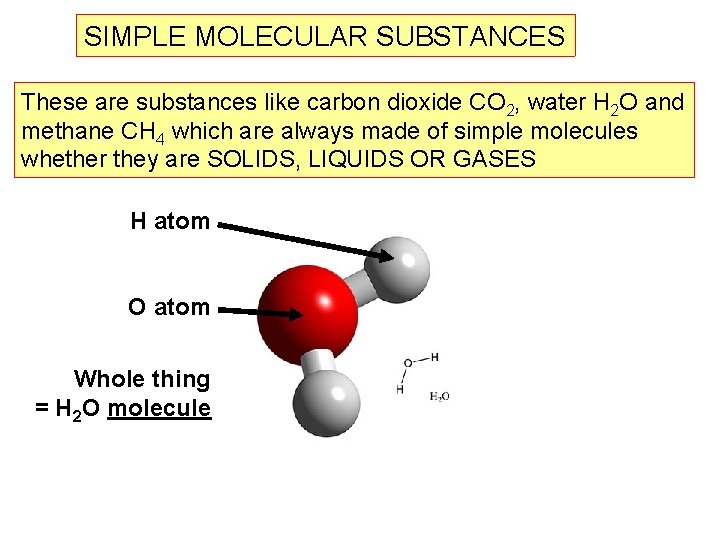
SIMPLE MOLECULAR SUBSTANCES These are substances like carbon dioxide CO 2, water H 2 O and methane CH 4 which are always made of simple molecules whether they are SOLIDS, LIQUIDS OR GASES H atom O atom Whole thing = H 2 O molecule
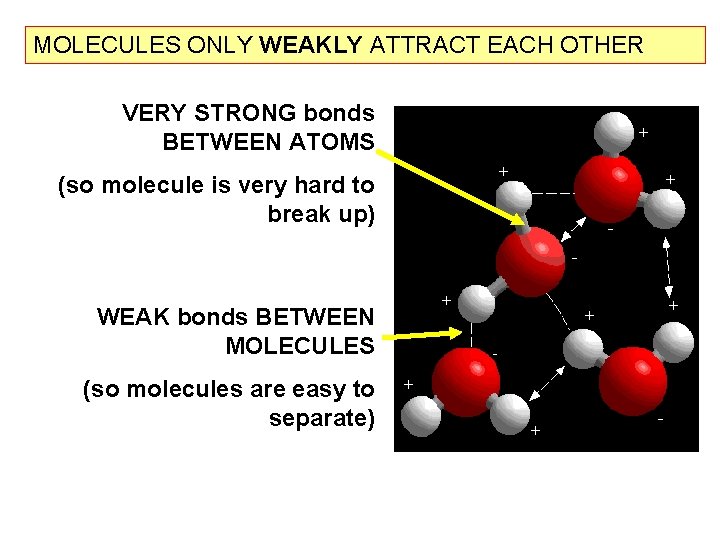
MOLECULES ONLY WEAKLY ATTRACT EACH OTHER VERY STRONG bonds BETWEEN ATOMS (so molecule is very hard to break up) WEAK bonds BETWEEN MOLECULES (so molecules are easy to separate)
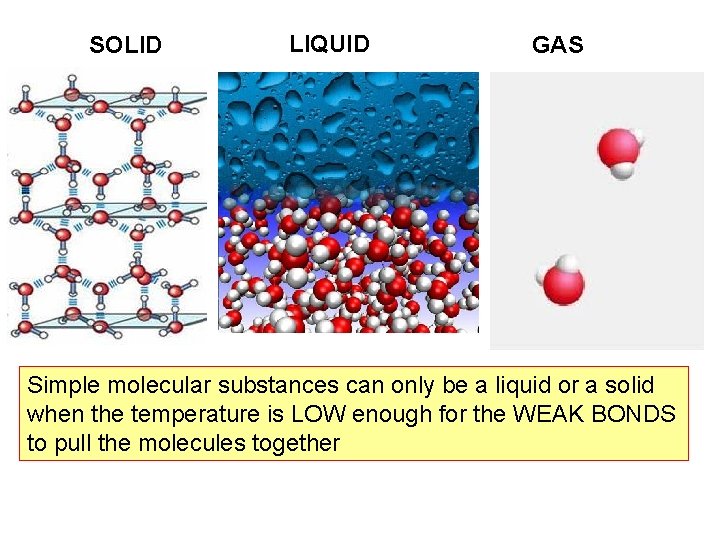
SOLID LIQUID GAS Simple molecular substances can only be a liquid or a solid when the temperature is LOW enough for the WEAK BONDS to pull the molecules together
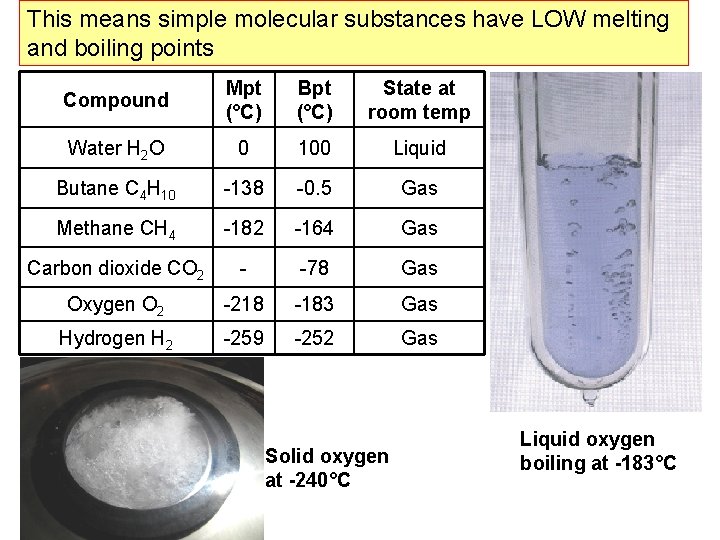
This means simple molecular substances have LOW melting and boiling points Compound Mpt (°C) Bpt (°C) State at room temp Water H 2 O 0 100 Liquid Butane C 4 H 10 -138 -0. 5 Gas Methane CH 4 -182 -164 Gas Carbon dioxide CO 2 - -78 Gas Oxygen O 2 -218 -183 Gas Hydrogen H 2 -259 -252 Gas Solid oxygen at -240°C Liquid oxygen boiling at -183°C
As the bonds between the molecules are weak, simple molecular substances are weak and soft when solid. As the molecules are NOT CHARGED simple molecular substances DON’T CONDUCT ELECTRICITY when solids, liquids or gases.
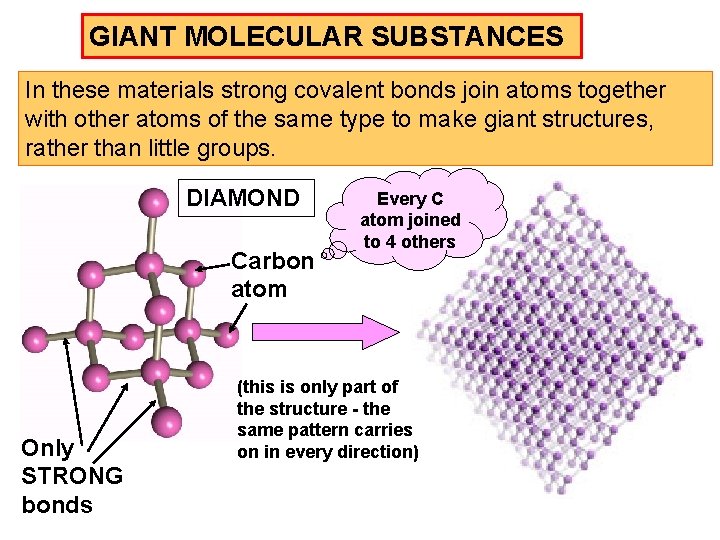
GIANT MOLECULAR SUBSTANCES In these materials strong covalent bonds join atoms together with other atoms of the same type to make giant structures, rather than little groups. DIAMOND Carbon atom Only STRONG bonds Every C atom joined to 4 others (this is only part of the structure - the same pattern carries on in every direction)
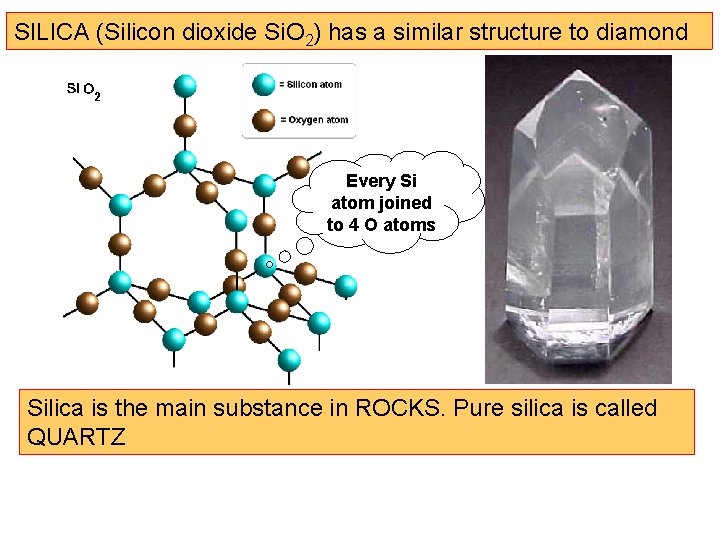
SILICA (Silicon dioxide Si. O 2) has a similar structure to diamond Every Si atom joined to 4 O atoms Silica is the main substance in ROCKS. Pure silica is called QUARTZ
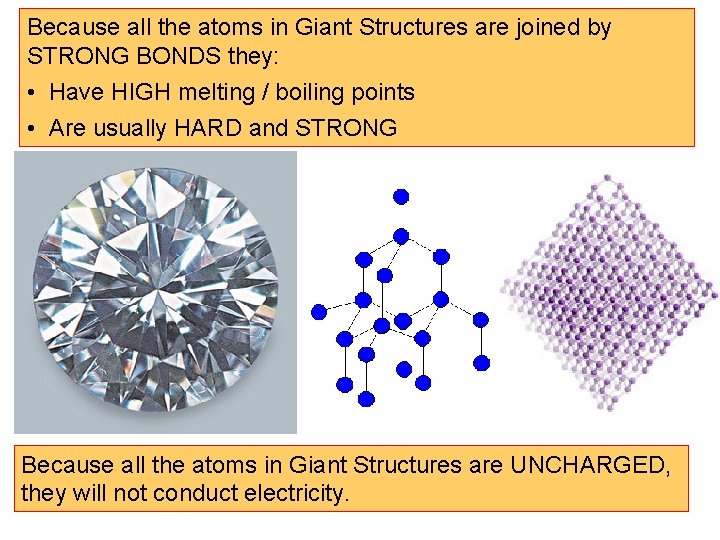
Because all the atoms in Giant Structures are joined by STRONG BONDS they: • Have HIGH melting / boiling points • Are usually HARD and STRONG Because all the atoms in Giant Structures are UNCHARGED, they will not conduct electricity.
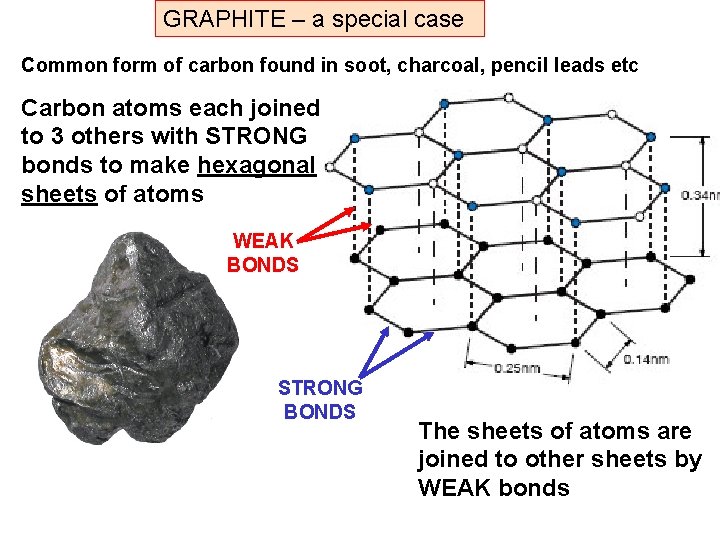
GRAPHITE – a special case Common form of carbon found in soot, charcoal, pencil leads etc Carbon atoms each joined to 3 others with STRONG bonds to make hexagonal sheets of atoms WEAK BONDS STRONG BONDS The sheets of atoms are joined to other sheets by WEAK bonds
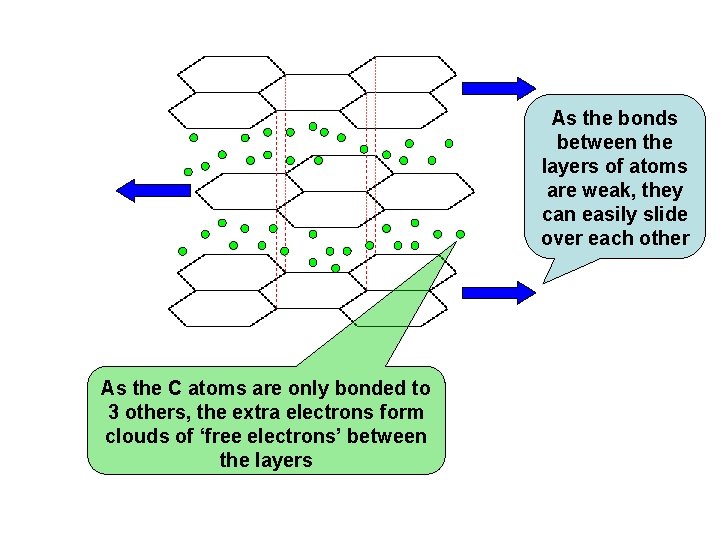
As the bonds between the layers of atoms are weak, they can easily slide over each other As the C atoms are only bonded to 3 others, the extra electrons form clouds of ‘free electrons’ between the layers
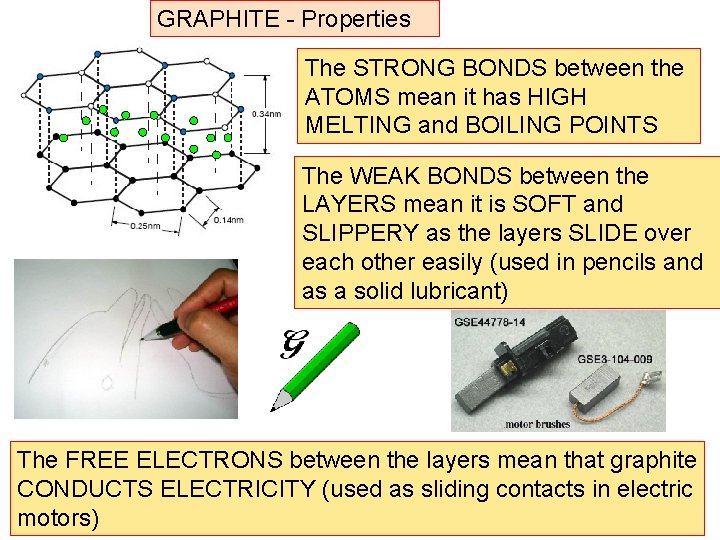
GRAPHITE - Properties The STRONG BONDS between the ATOMS mean it has HIGH MELTING and BOILING POINTS The WEAK BONDS between the LAYERS mean it is SOFT and SLIPPERY as the layers SLIDE over each other easily (used in pencils and as a solid lubricant) The FREE ELECTRONS between the layers mean that graphite CONDUCTS ELECTRICITY (used as sliding contacts in electric motors)
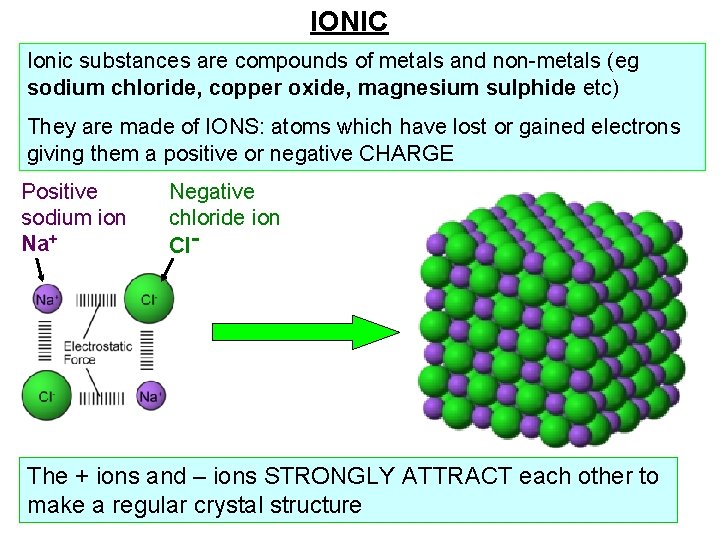
IONIC Ionic substances are compounds of metals and non-metals (eg sodium chloride, copper oxide, magnesium sulphide etc) They are made of IONS: atoms which have lost or gained electrons giving them a positive or negative CHARGE Positive sodium ion Na+ Negative chloride ion Cl- The + ions and – ions STRONGLY ATTRACT each other to make a regular crystal structure
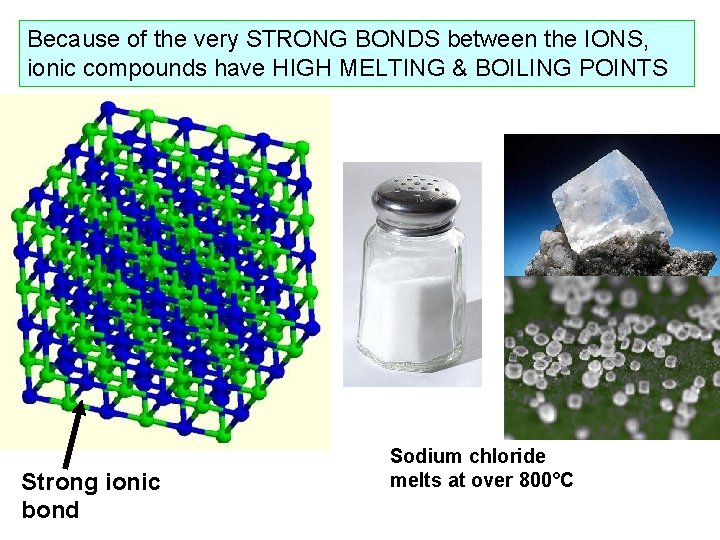
Because of the very STRONG BONDS between the IONS, ionic compounds have HIGH MELTING & BOILING POINTS Strong ionic bond Sodium chloride melts at over 800°C
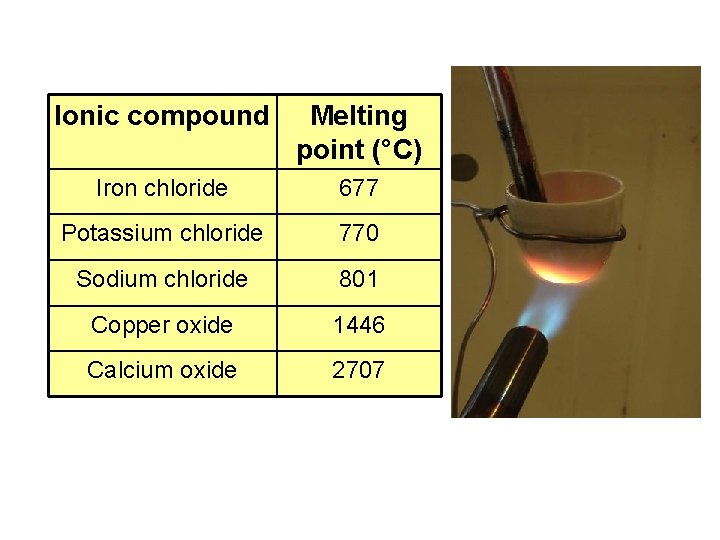
Ionic compound Melting point (°C) Iron chloride 677 Potassium chloride 770 Sodium chloride 801 Copper oxide 1446 Calcium oxide 2707
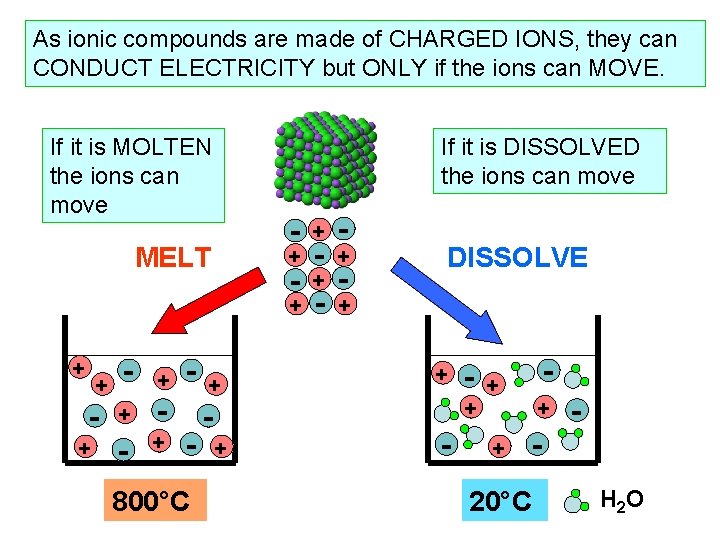
As ionic compounds are made of CHARGED IONS, they can CONDUCT ELECTRICITY but ONLY if the ions can MOVE. If it is MOLTEN the ions can move MELT + - + - + - + + 800°C If it is DISSOLVED the ions can move - + + - - + - DISSOLVE + - + + + - - - + 20°C H 2 O
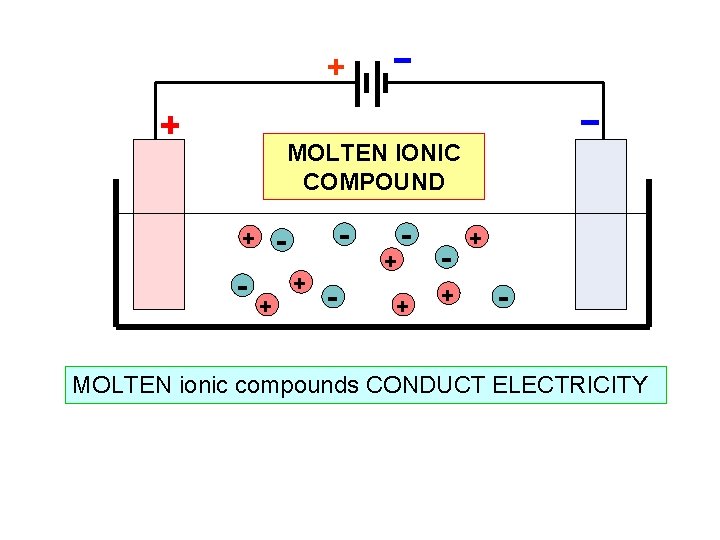
+ MOLTEN IONIC COMPOUND - + - - + + + - MOLTEN ionic compounds CONDUCT ELECTRICITY
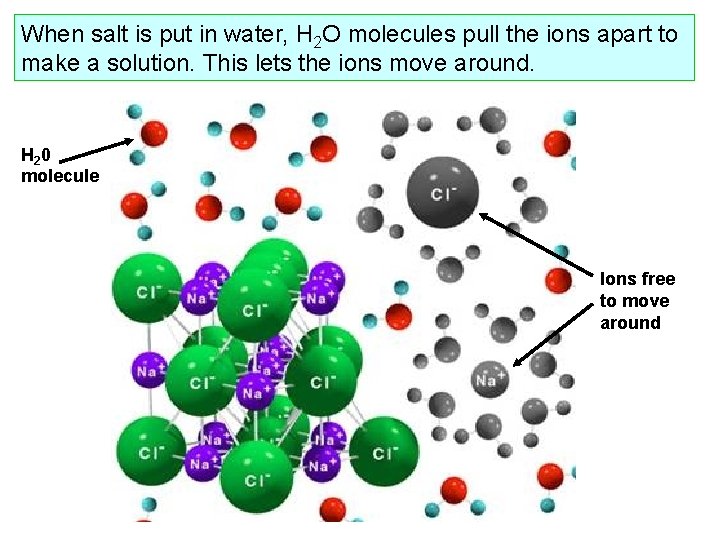
When salt is put in water, H 2 O molecules pull the ions apart to make a solution. This lets the ions move around. H 2 0 molecule Ions free to move around
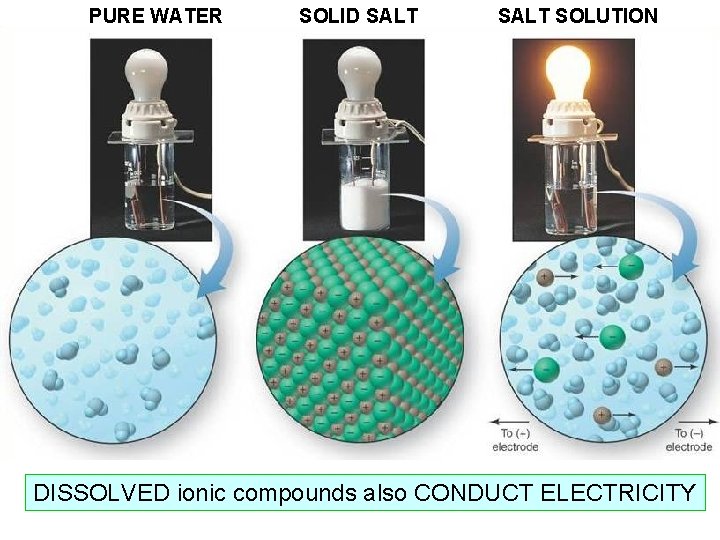
PURE WATER SOLID SALT SOLUTION DISSOLVED ionic compounds also CONDUCT ELECTRICITY

METALS In a metal the atoms are held together by strong bonds in regular structures. This means most metals have high melting and boiling points and are hard and strong
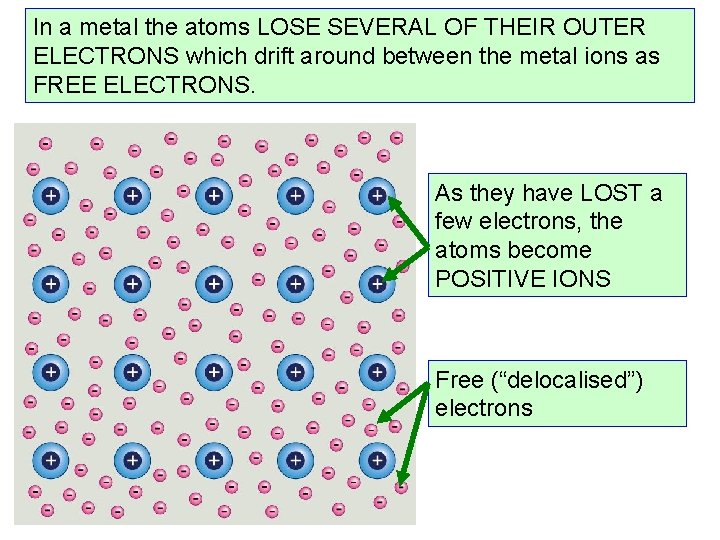
In a metal the atoms LOSE SEVERAL OF THEIR OUTER ELECTRONS which drift around between the metal ions as FREE ELECTRONS. As they have LOST a few electrons, the atoms become POSITIVE IONS Free (“delocalised”) electrons
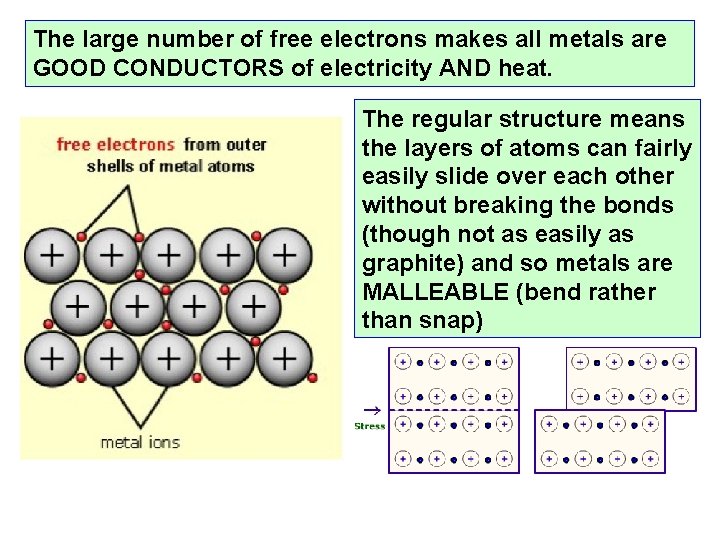
The large number of free electrons makes all metals are GOOD CONDUCTORS of electricity AND heat. The regular structure means the layers of atoms can fairly easily slide over each other without breaking the bonds (though not as easily as graphite) and so metals are MALLEABLE (bend rather than snap)
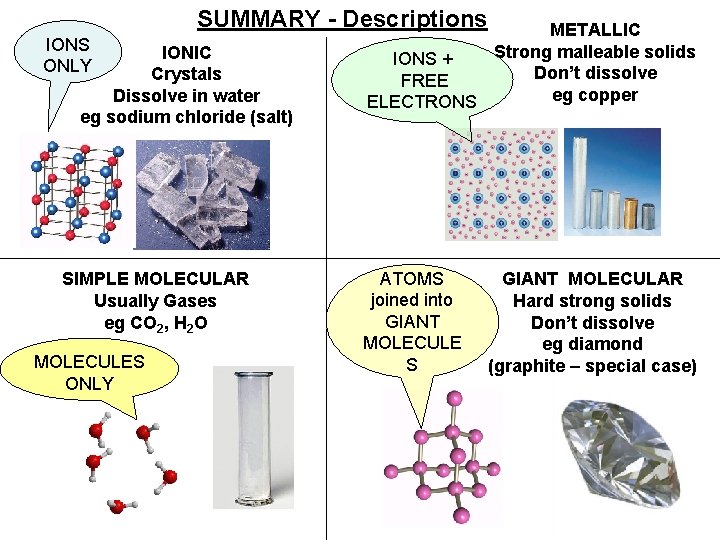
SUMMARY - Descriptions IONS ONLY IONIC Crystals Dissolve in water eg sodium chloride (salt) SIMPLE MOLECULAR Usually Gases eg CO 2, H 2 O MOLECULES ONLY METALLIC Strong malleable solids IONS + Don’t dissolve FREE eg copper ELECTRONS ATOMS joined into GIANT MOLECULE S GIANT MOLECULAR Hard strong solids Don’t dissolve eg diamond (graphite – special case)
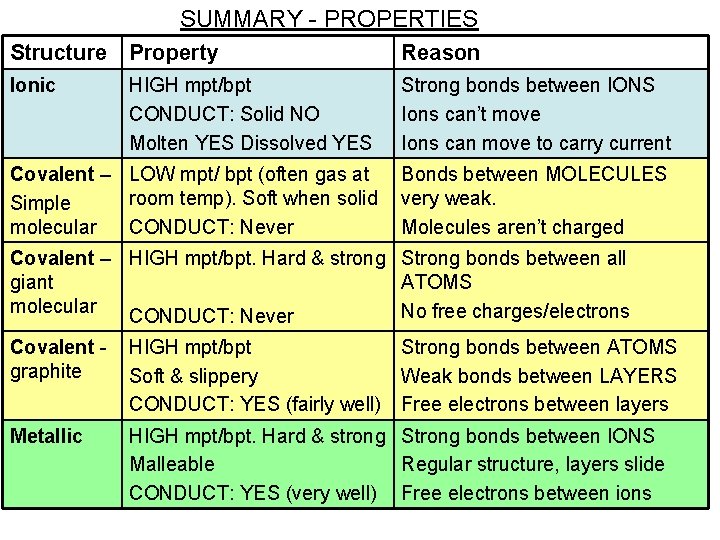
SUMMARY - PROPERTIES Structure Property Reason Ionic HIGH mpt/bpt CONDUCT: Solid NO Molten YES Dissolved YES Strong bonds between IONS Ions can’t move Ions can move to carry current Covalent – LOW mpt/ bpt (often gas at room temp). Soft when solid Simple molecular CONDUCT: Never Bonds between MOLECULES very weak. Molecules aren’t charged Covalent – HIGH mpt/bpt. Hard & strong Strong bonds between all giant ATOMS molecular No free charges/electrons CONDUCT: Never Covalent graphite HIGH mpt/bpt Soft & slippery CONDUCT: YES (fairly well) Strong bonds between ATOMS Weak bonds between LAYERS Free electrons between layers Metallic HIGH mpt/bpt. Hard & strong Strong bonds between IONS Malleable Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions
- Slides: 26