Structures and Properties of Covalent Compounds Small covalent
- Slides: 8

Structures and Properties of Covalent Compounds

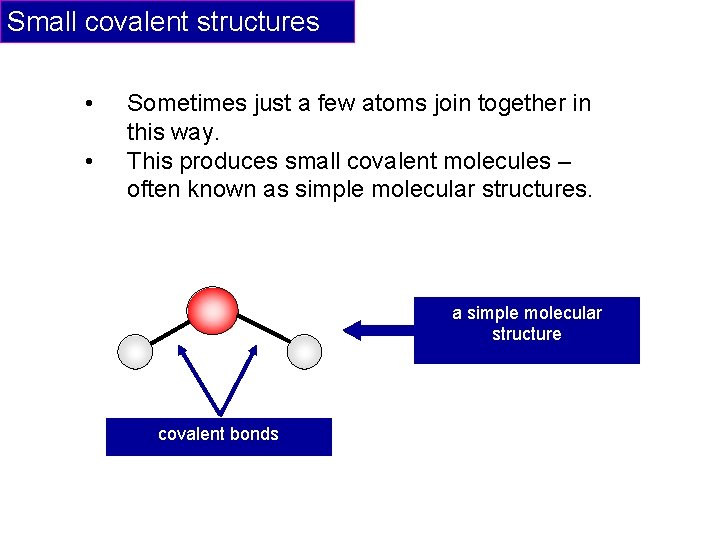
Small covalent structures • • Sometimes just a few atoms join together in this way. This produces small covalent molecules – often known as simple molecular structures. a simple molecular structure covalent bonds

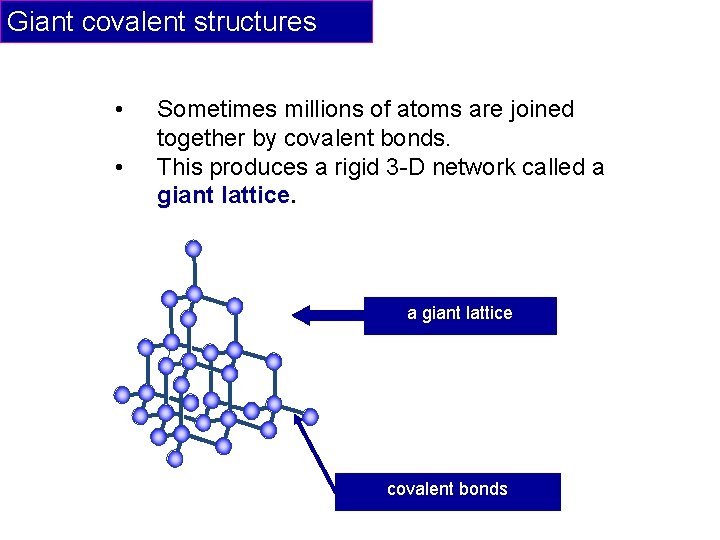
Giant covalent structures • • Sometimes millions of atoms are joined together by covalent bonds. This produces a rigid 3 -D network called a giant lattice covalent bonds

Giant covalent structures 1. Carbon atoms form giant structures. 2. What is interesting is that there is more than one possible arrangement for the atoms. 3. Although this does not affect the chemical properties it can make a huge difference to the physical properties such as hardness, slipperiness, melting point and density. Different arrangements of the same element are called allotropes. C

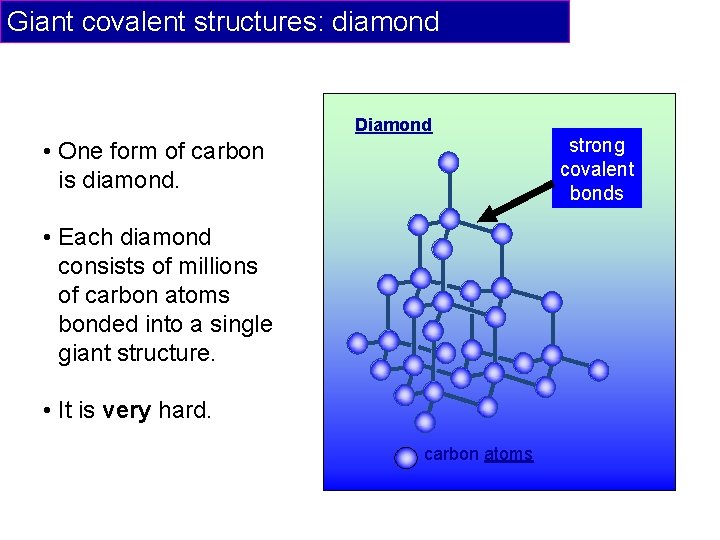
Giant covalent structures: diamond Diamond • One form of carbon is diamond. • Each diamond consists of millions of carbon atoms bonded into a single giant structure. • It is very hard. carbon atoms strong covalent bonds

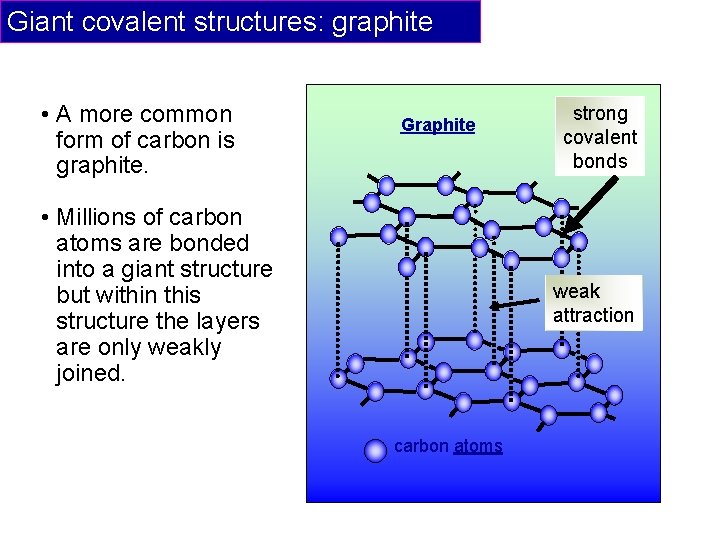
Giant covalent structures: graphite • A more common form of carbon is graphite. Graphite • Millions of carbon atoms are bonded into a giant structure but within this structure the layers are only weakly joined. strong covalent bonds weak attraction carbon atoms

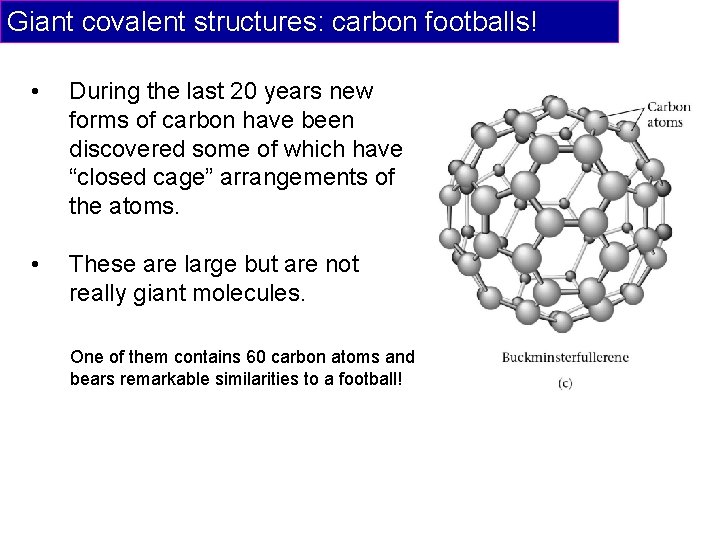
Giant covalent structures: carbon footballs! • During the last 20 years new forms of carbon have been discovered some of which have “closed cage” arrangements of the atoms. • These are large but are not really giant molecules. One of them contains 60 carbon atoms and bears remarkable similarities to a football!

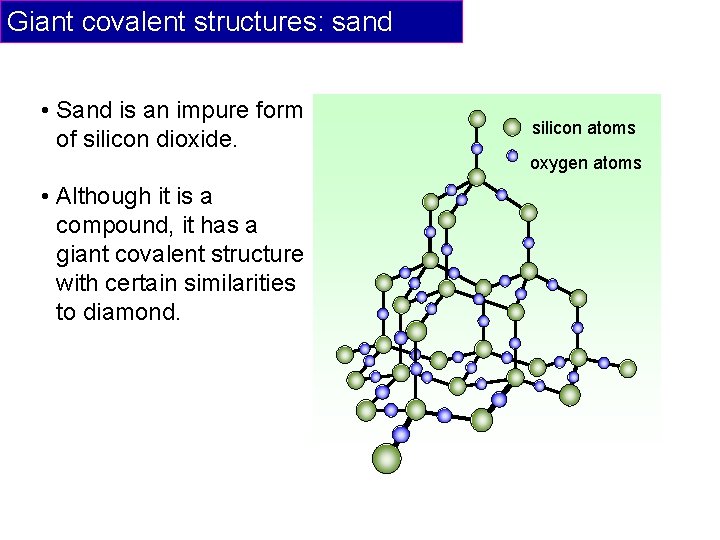
Giant covalent structures: sand • Sand is an impure form of silicon dioxide. silicon atoms oxygen atoms • Although it is a compound, it has a giant covalent structure with certain similarities to diamond.