Structure properties INTERMOLECULAR FORCES INTRAMOLECULAR FORCES Ionic bonds
Structure & properties INTERMOLECULAR FORCES
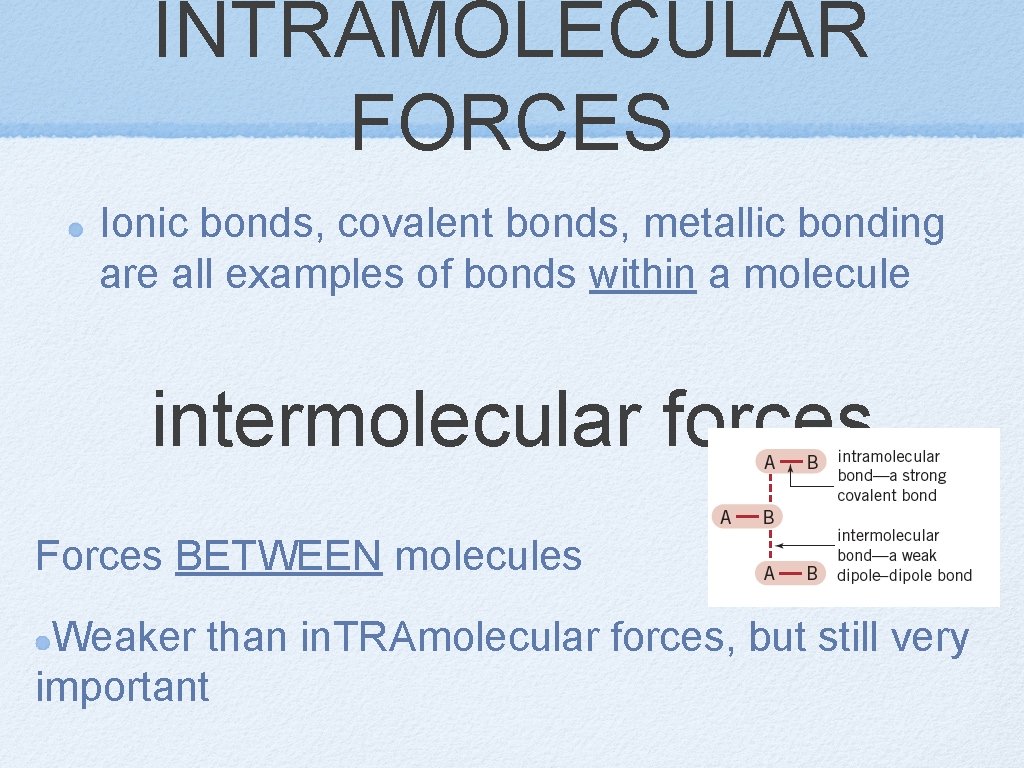
INTRAMOLECULAR FORCES Ionic bonds, covalent bonds, metallic bonding are all examples of bonds within a molecule intermolecular forces Forces BETWEEN molecules Weaker than in. TRAmolecular forces, but still very important

intermolecular forces! • Featuring the exciting world of. . . • Dipole dipole, hydrogen bonding, ion dipole, Van der Waal’s/London dispersion forces!
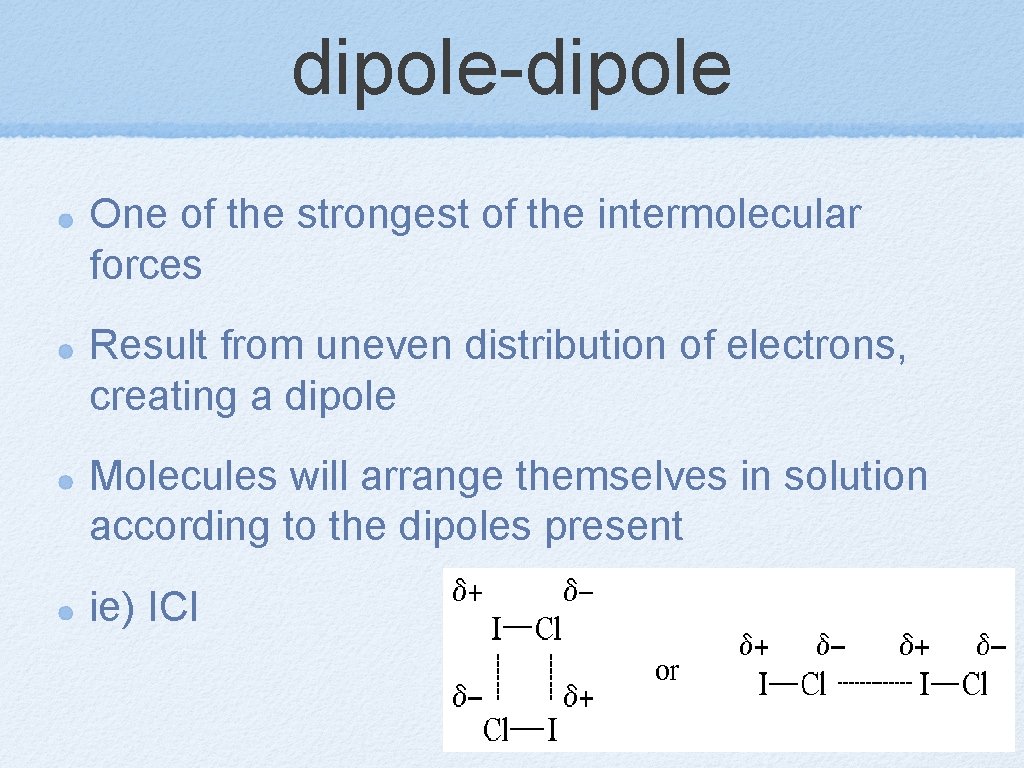
dipole-dipole One of the strongest of the intermolecular forces Result from uneven distribution of electrons, creating a dipole Molecules will arrange themselves in solution according to the dipoles present ie) ICl

impact on mp/bp Dipoles will INCREASE melting and boiling points The molecules are now stuck together more tightly (opposites attract!) and so more energy is required to melt/boil the substance
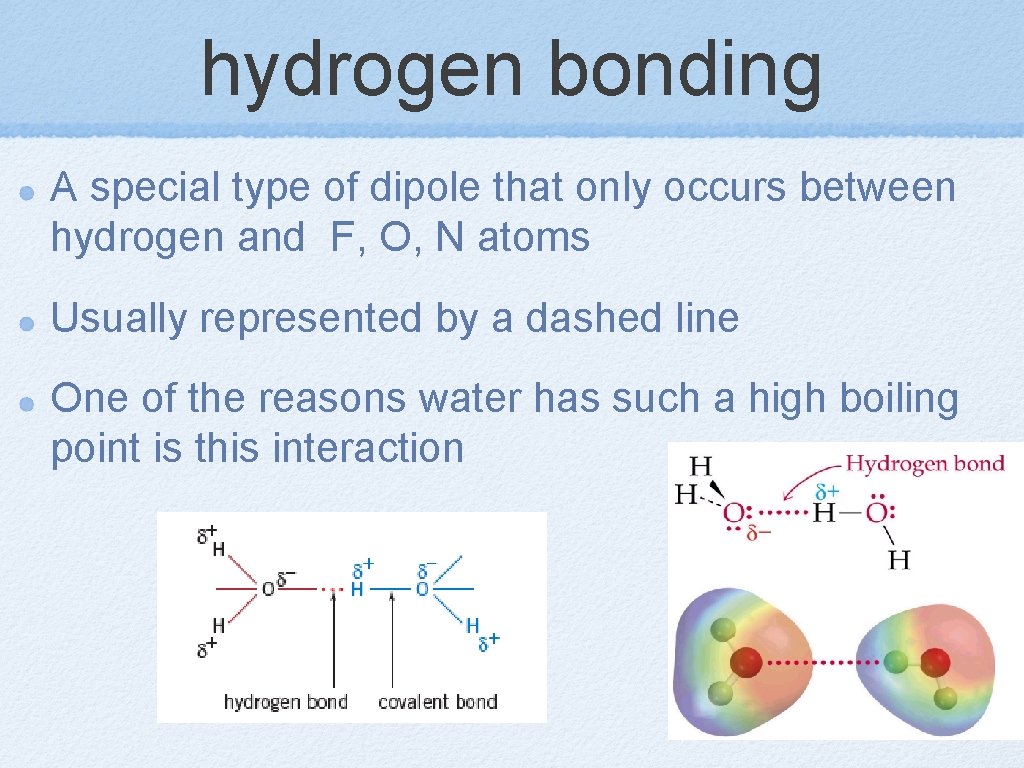
hydrogen bonding A special type of dipole that only occurs between hydrogen and F, O, N atoms Usually represented by a dashed line One of the reasons water has such a high boiling point is this interaction
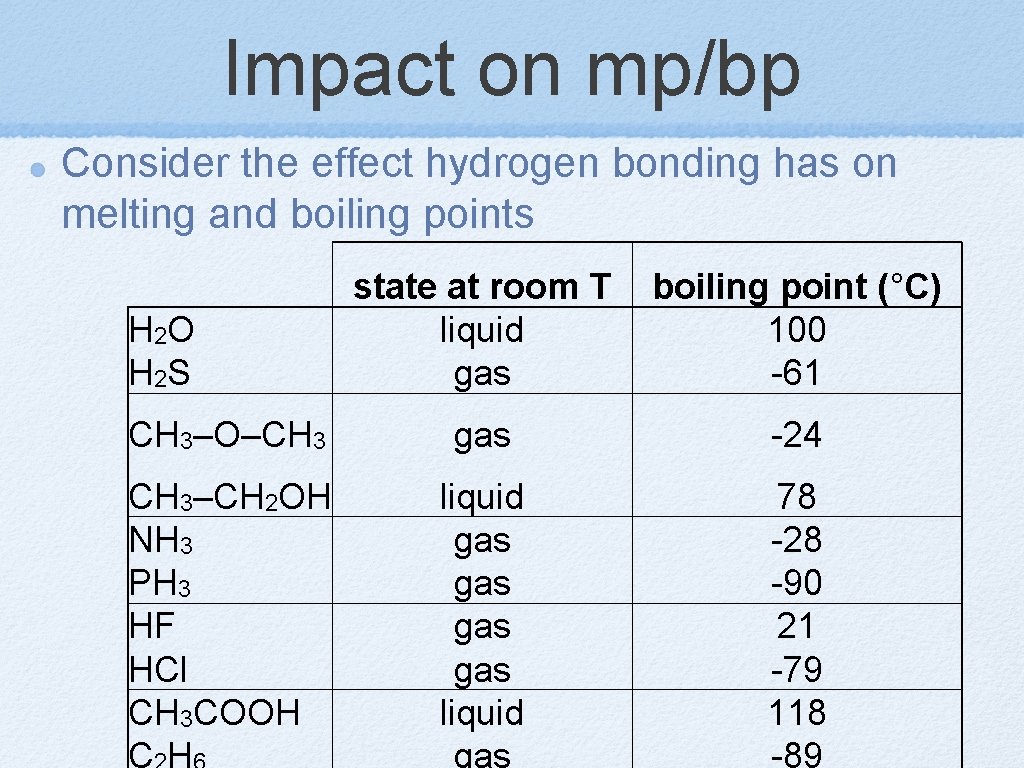
Impact on mp/bp Consider the effect hydrogen bonding has on melting and boiling points state at room T liquid gas boiling point (°C) 100 -61 CH 3–O–CH 3 gas -24 CH 3–CH 2 OH NH 3 PH 3 HF HCl CH 3 COOH CH liquid gas gas liquid gas 78 -28 -90 21 -79 118 -89 H 2 O H 2 S
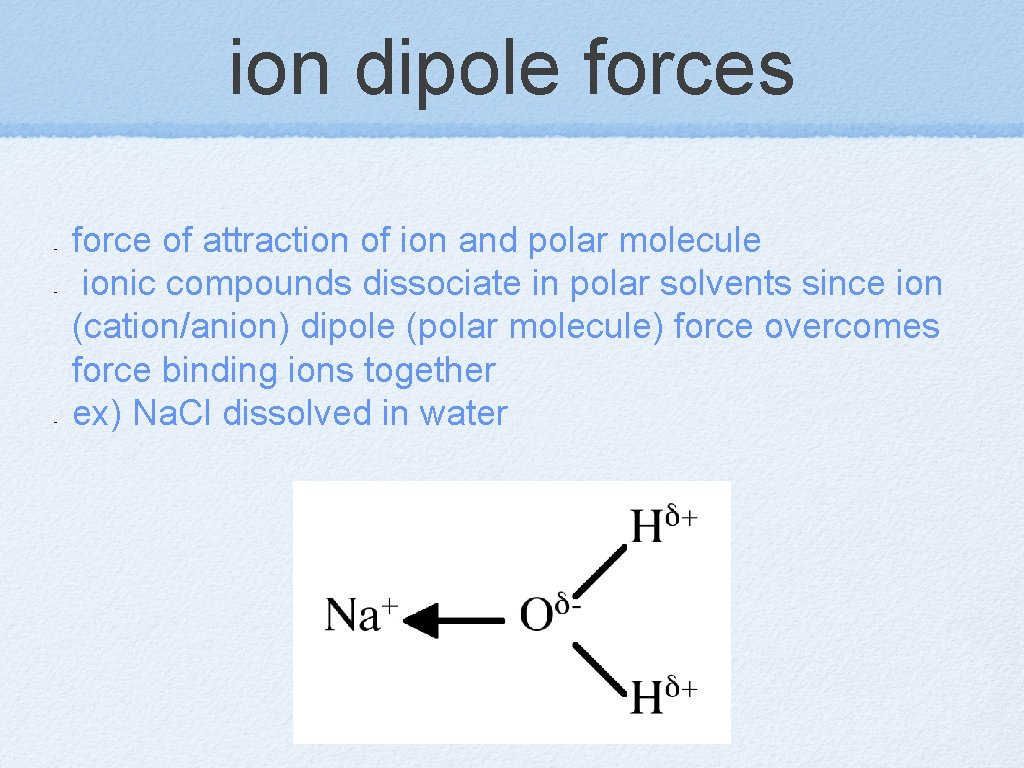
ion dipole forces - - - force of attraction of ion and polar molecule ionic compounds dissociate in polar solvents since ion (cation/anion) dipole (polar molecule) force overcomes force binding ions together ex) Na. Cl dissolved in water

Think about it! • • You are making some pasta. The instructions indicate you should boil the pasta in salted water. You are in a rush and want to eat as quickly as possible! Should you add salt BEFORE you boil the water or AFTER the water has started to boil? Explain why!
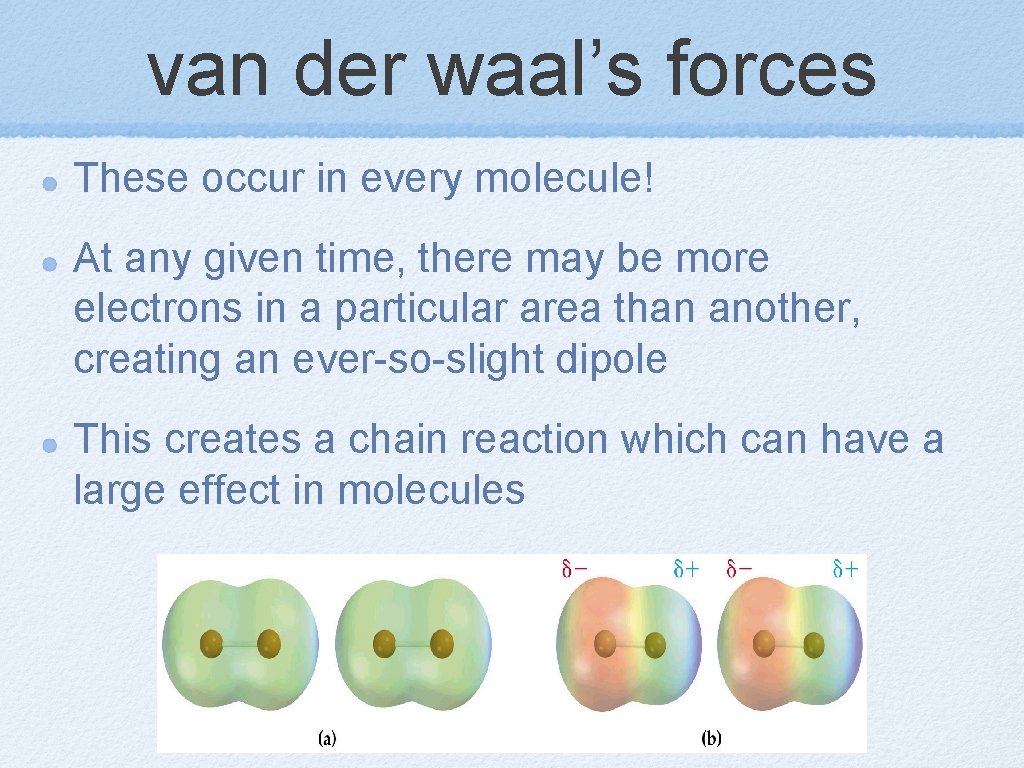
van der waal’s forces These occur in every molecule! At any given time, there may be more electrons in a particular area than another, creating an ever-so-slight dipole This creates a chain reaction which can have a large effect in molecules
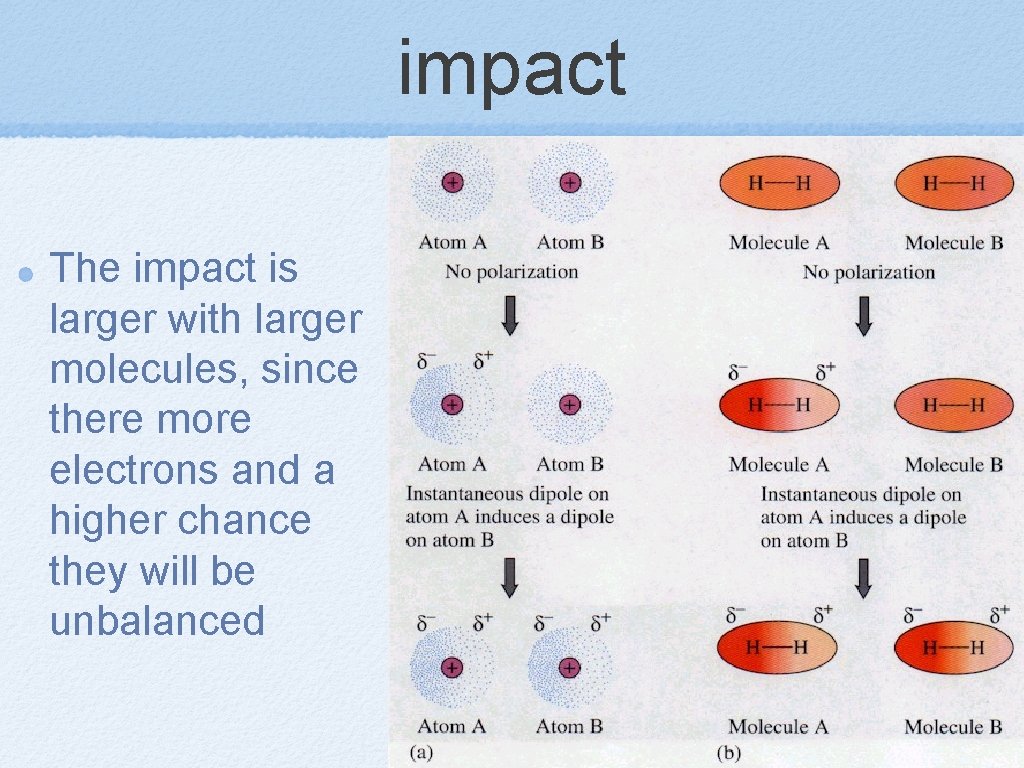
impact The impact is larger with larger molecules, since there more electrons and a higher chance they will be unbalanced


Who has the highest bp? H 2 O vs H 2 S H 2 O due to hydrogen bonding, NH 3 vs PH 3 NH 3 due to hydrogen bonding CHCl 3 vs CH 4 CHCl 3 due to dipole-dipole CH 4 vs C 10 H 20 due to larger VDW forces

try it!

try it! Nelson Chemistry 12 Read p. 257 -265 Try p. 260 #1 -5; p. 264 #9, 11
- Slides: 15