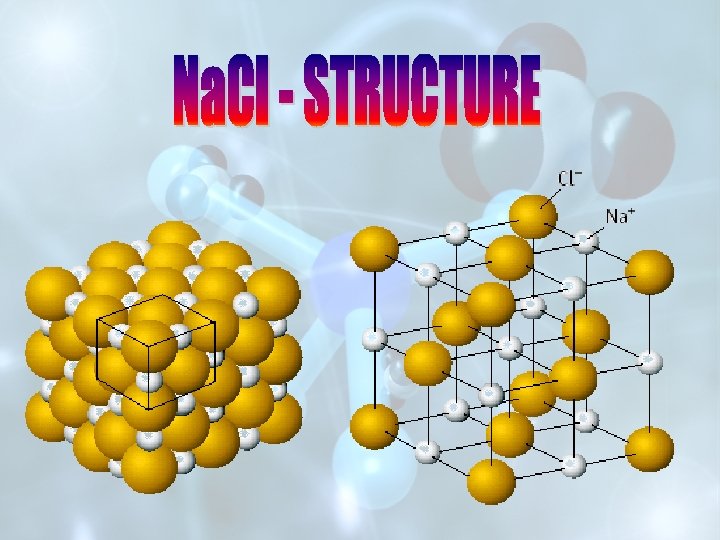
STRUCTURE OF Na Cl SOLID STATE DEFECTS IN






- Slides: 34


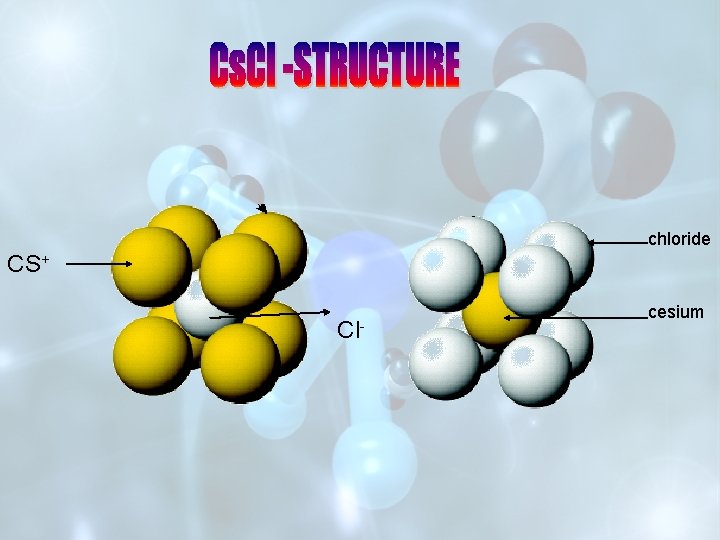
STRUCTURE OF Na. Cl SOLID STATE DEFECTS IN SOLIDS STRUCTURE OF Cs. Cl




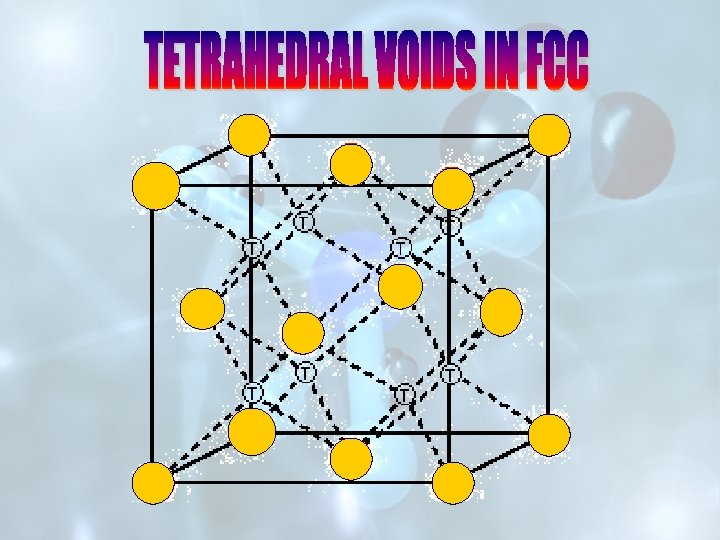
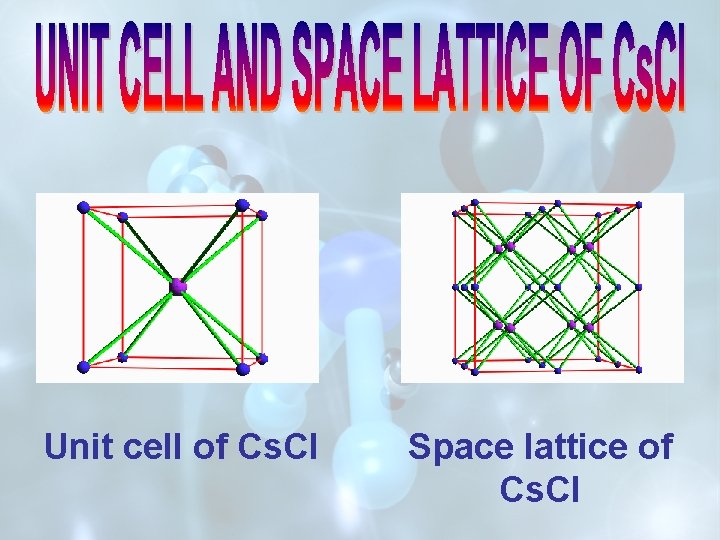
UNIT CELL OF Na. Cl OR SPACE LATTICE OF Cs. Cl


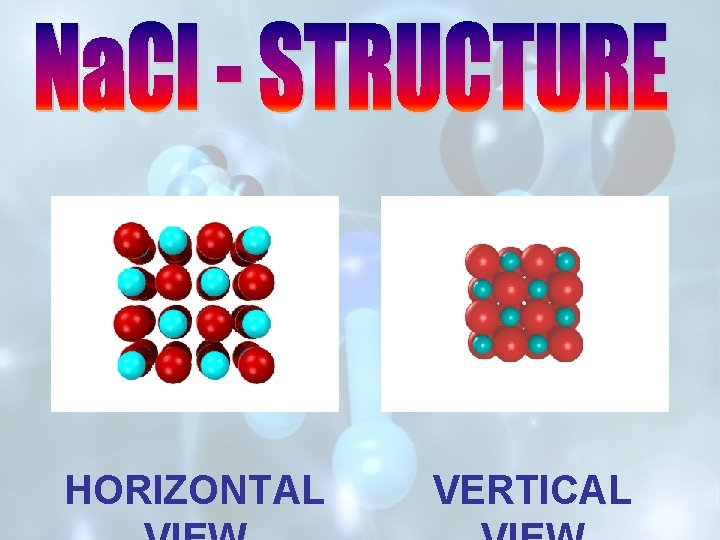
HORIZONTAL VERTICAL

FRONT VIEW OF UNIT CELL 3 – D VIEW OF UNIT CELL OF Na. Cl

chloride CS+ Cl- cesium


Unit cell of Cs. Cl Space lattice of Cs. Cl


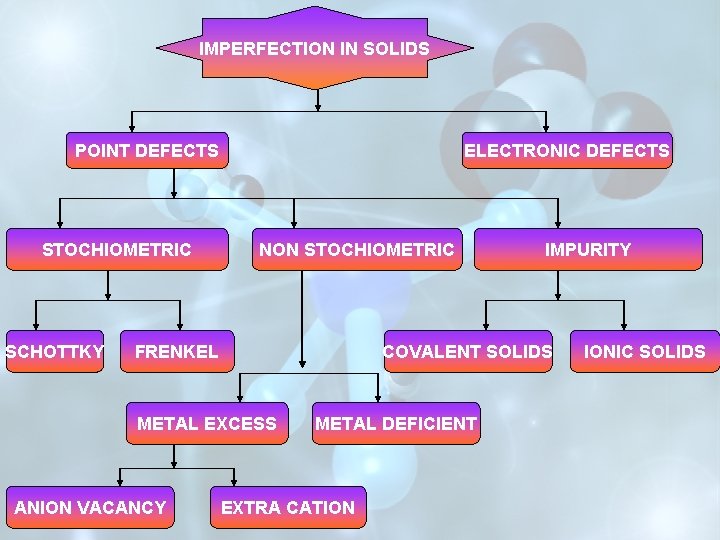
IMPERFECTION IN SOLIDS POINT DEFECTS STOCHIOMETRIC SCHOTTKY ELECTRONIC DEFECTS NON STOCHIOMETRIC COVALENT SOLIDS FRENKEL METAL EXCESS ANION VACANCY IMPURITY METAL DEFICIENT EXTRA CATION IONIC SOLIDS

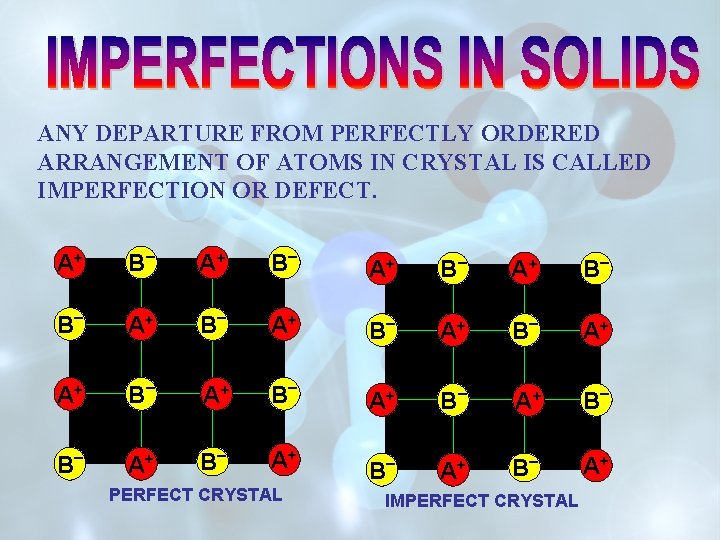
ANY DEPARTURE FROM PERFECTLY ORDERED ARRANGEMENT OF ATOMS IN CRYSTAL IS CALLED IMPERFECTION OR DEFECT. A+ B _ B _ A+ A+ B _ A+ B PERFECT CRYSTAL A+ B _ B _ A+ A+ B _ IMPERFECT CRYSTAL B _ A+

STOICHIOMETRIC NON-STOICHIOMETRIC IMPURITY DEFECT

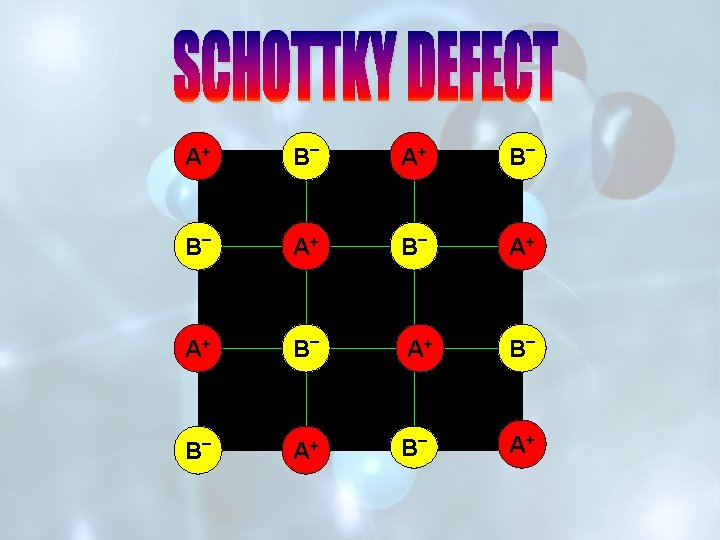
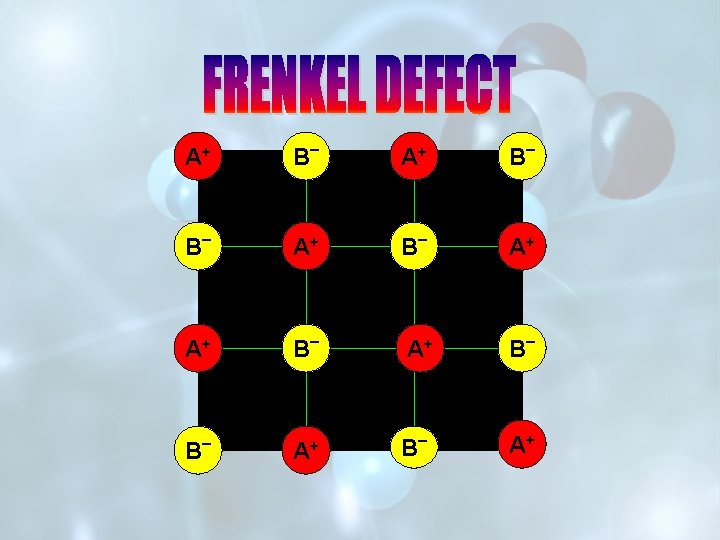
IF IMPERFECTIONS IN THE CRYSTAL ARE SUCH THAT THE RATIO BETWEEN THE CATIONS AND ANIONS REMAINS THE SAME AS REPRESENTED BY THE MOLECULAR FORMULA, THE DEFECTS ARE CALLED STOICHIOMETRIC DEFECTS. v. SCHOTTKY DEFECTS v. FRENKEL DEFECTS



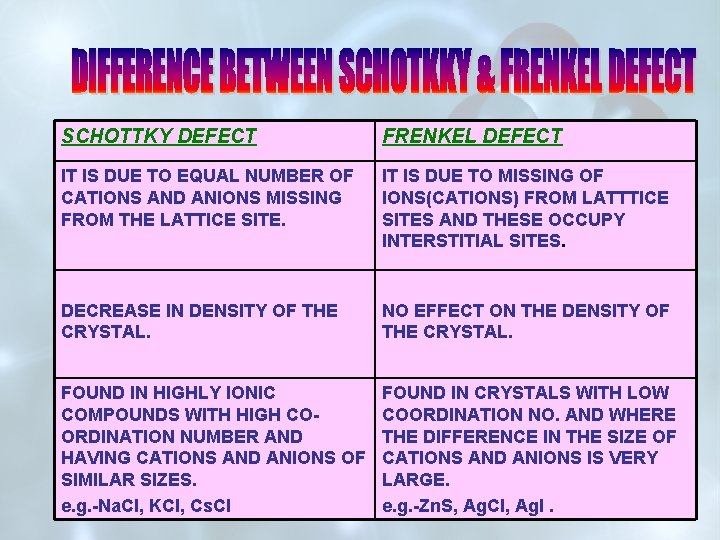
SCHOTTKY DEFECT FRENKEL DEFECT IT IS DUE TO EQUAL NUMBER OF CATIONS AND ANIONS MISSING FROM THE LATTICE SITE. IT IS DUE TO MISSING OF IONS(CATIONS) FROM LATTTICE SITES AND THESE OCCUPY INTERSTITIAL SITES. DECREASE IN DENSITY OF THE CRYSTAL. NO EFFECT ON THE DENSITY OF THE CRYSTAL. FOUND IN HIGHLY IONIC COMPOUNDS WITH HIGH COORDINATION NUMBER AND HAVING CATIONS AND ANIONS OF SIMILAR SIZES. e. g. -Na. Cl, KCl, Cs. Cl FOUND IN CRYSTALS WITH LOW COORDINATION NO. AND WHERE THE DIFFERENCE IN THE SIZE OF CATIONS AND ANIONS IS VERY LARGE. e. g. -Zn. S, Ag. Cl, Ag. I.

IF AS A RESULT OF THE IMPERFECTIONS IN THE CRYSTAL THE RATIO OF THE CATIONS TO THE ANION BECOMES DIFFERENT FROM THAT INDICATED BY THE IDEAL CHEMICAL FORMULA, THE DEFECTS ARE CALLED NONSTOICIOMETRIC DEFECTS. (A)METAL EXCESS (B)METAL DEFICIENCY

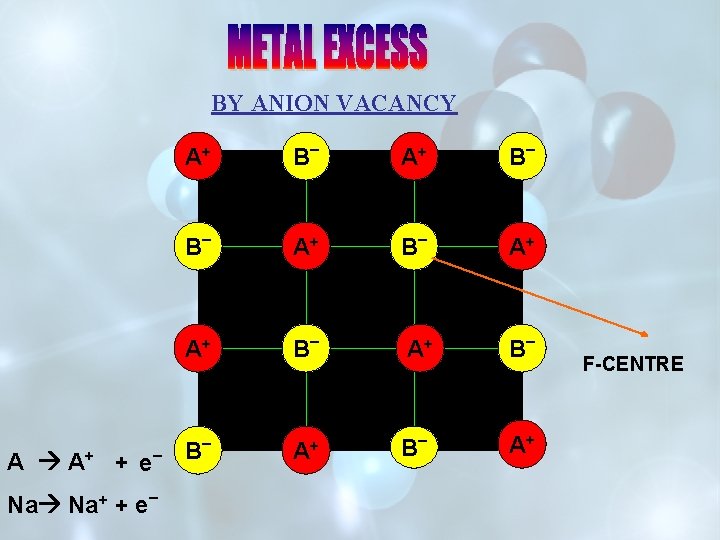
BY ANION VACANCY A+ B _ A+ A A+ + e Na Na+ +e _ _ B _ A+ A+ B _ A+ B F-CENTRE

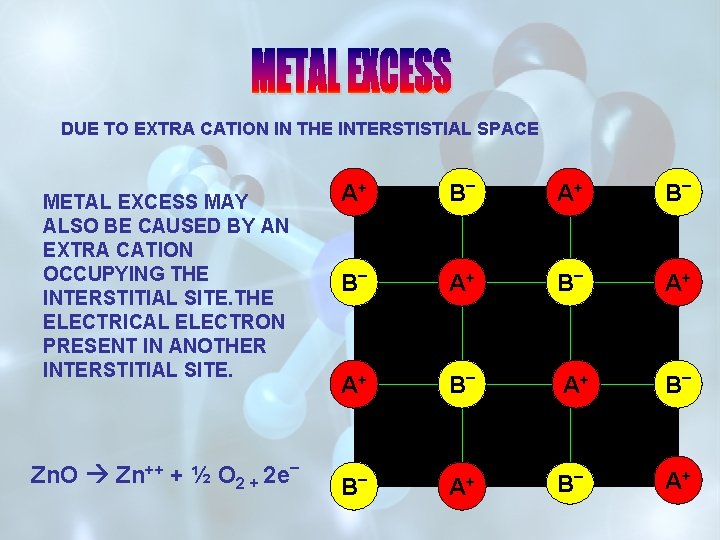
DUE TO EXTRA CATION IN THE INTERSTISTIAL SPACE A+ METAL EXCESS MAY ALSO BE CAUSED BY AN EXTRA CATION OCCUPYING THE INTERSTITIAL SITE. THE ELECTRICAL ELECTRON PRESENT IN ANOTHER INTERSTITIAL SITE. Zn. O Zn++ + ½ O 2 + 2 e B _ A+ _ B _ A+ A+ B _ A+ B

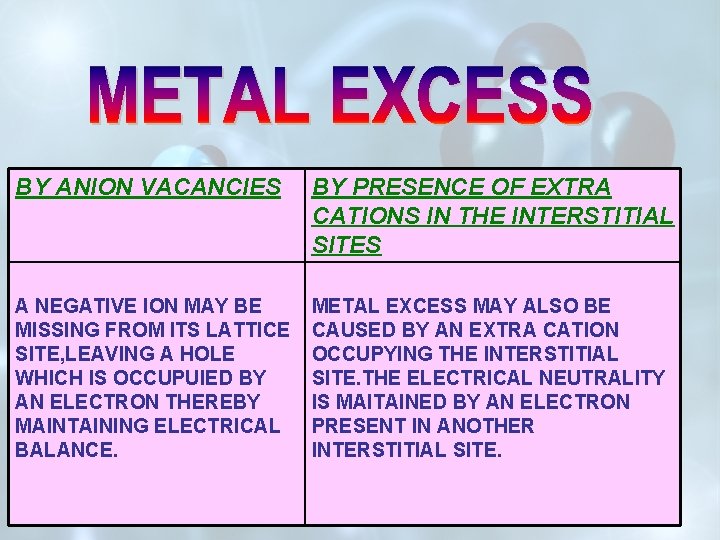
BY ANION VACANCIES BY PRESENCE OF EXTRA CATIONS IN THE INTERSTITIAL SITES A NEGATIVE ION MAY BE MISSING FROM ITS LATTICE SITE, LEAVING A HOLE WHICH IS OCCUPUIED BY AN ELECTRON THEREBY MAINTAINING ELECTRICAL BALANCE. METAL EXCESS MAY ALSO BE CAUSED BY AN EXTRA CATION OCCUPYING THE INTERSTITIAL SITE. THE ELECTRICAL NEUTRALITY IS MAITAINED BY AN ELECTRON PRESENT IN ANOTHER INTERSTITIAL SITE.

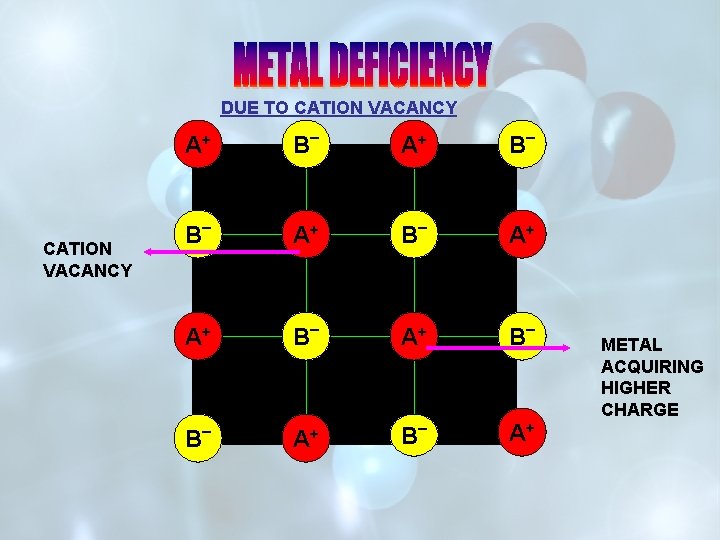
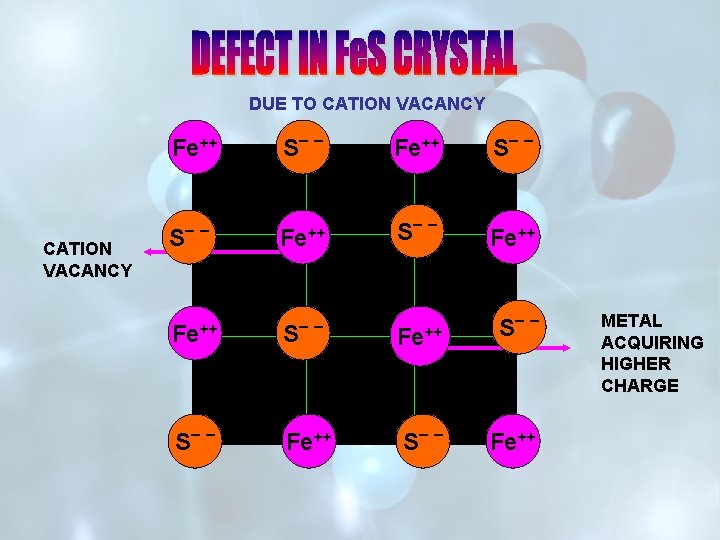
DUE TO CATION VACANCY A+ CATION VACANCY B _ A+ A+ B _ + A A++ B _ A+ METAL ACQUIRING HIGHER CHARGE

DUE TO CATION VACANCY Fe++ CATION VACANCY S _ _ Fe++ S _ _ ++ Fe Fe+++ S _ _ Fe++ METAL ACQUIRING HIGHER CHARGE

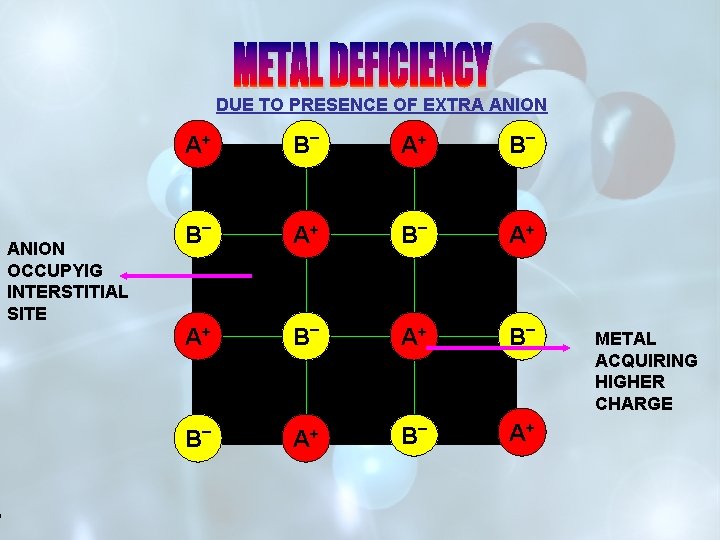
DUE TO PRESENCE OF EXTRA ANION A+ ANION OCCUPYIG INTERSTITIAL SITE B _ A+ A+ B _ + A A++ B _ A+ METAL ACQUIRING HIGHER CHARGE

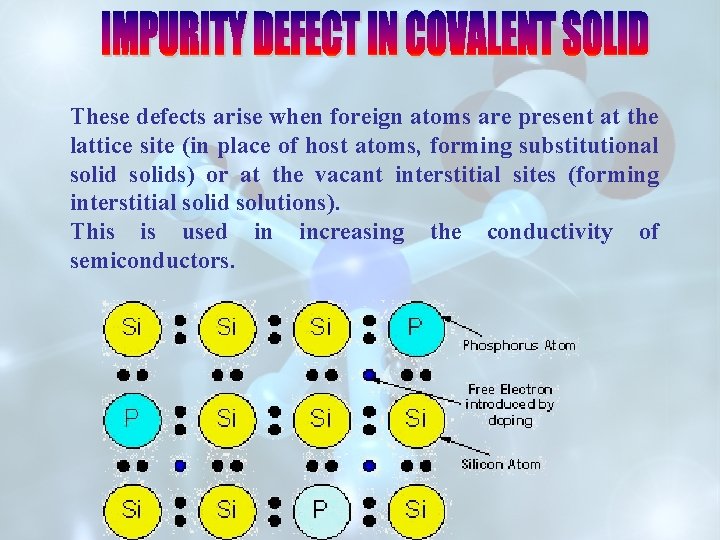
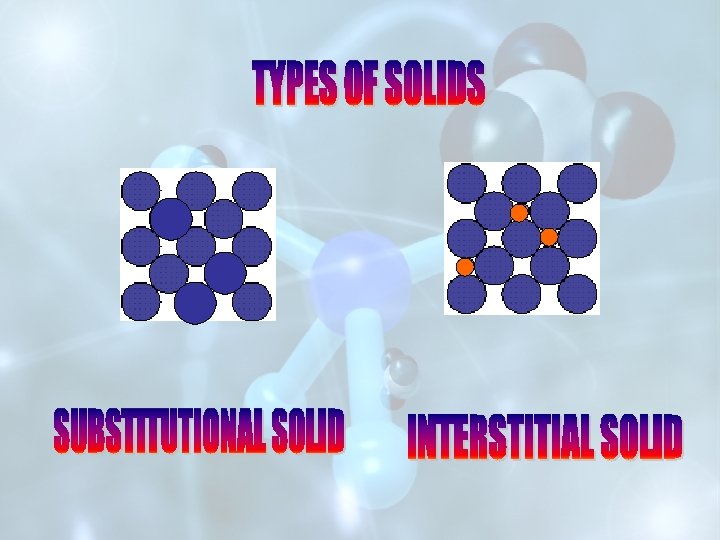
THESE DEFECTS ARISE WHEN FOREIGN ATOMS ARE PRESENT AT THE LATTICE SITE(IN PLACE OF HOST ATOMS) OR AT THE VACANT INTERSTITIAL SITES. (A) INTRODUCING IMPURITY DEFECT IN COVALENT SOLIDS (B) INTRODUCING IMPURITY DEFECT IN IONIC SOLIDS

These defects arise when foreign atoms are present at the lattice site (in place of host atoms, forming substitutional solids) or at the vacant interstitial sites (forming interstitial solid solutions). This is used in increasing the conductivity of semiconductors.


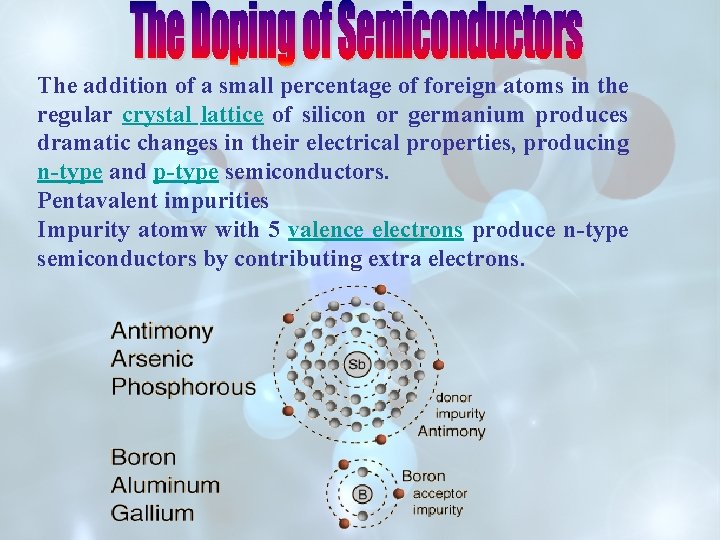
The addition of a small percentage of foreign atoms in the regular crystal lattice of silicon or germanium produces dramatic changes in their electrical properties, producing n-type and p-type semiconductors. Pentavalent impurities Impurity atomw with 5 valence electrons produce n-type semiconductors by contributing extra electrons.

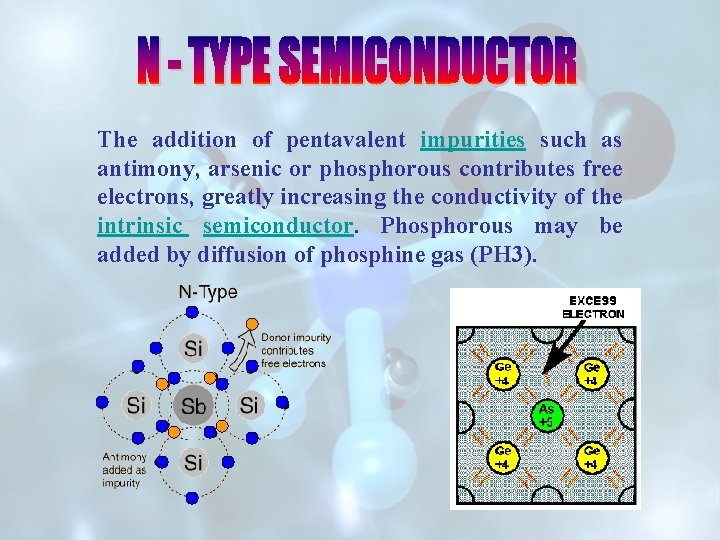
The addition of pentavalent impurities such as antimony, arsenic or phosphorous contributes free electrons, greatly increasing the conductivity of the intrinsic semiconductor. Phosphorous may be added by diffusion of phosphine gas (PH 3).

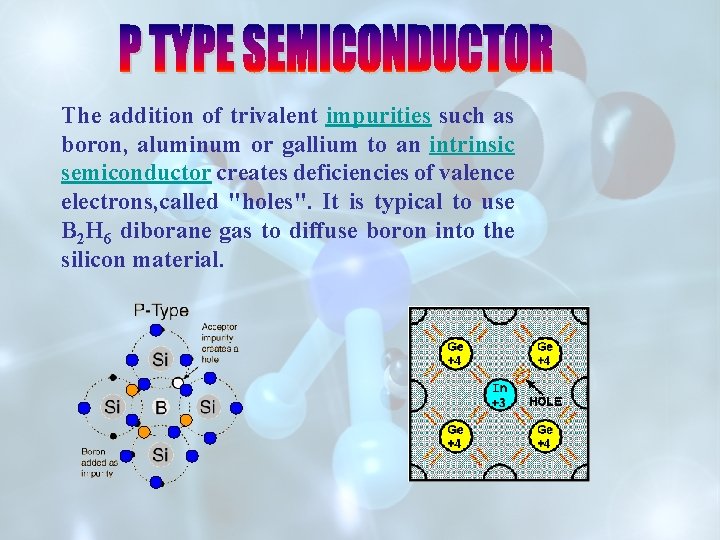
The addition of trivalent impurities such as boron, aluminum or gallium to an intrinsic semiconductor creates deficiencies of valence electrons, called "holes". It is typical to use B 2 H 6 diborane gas to diffuse boron into the silicon material.

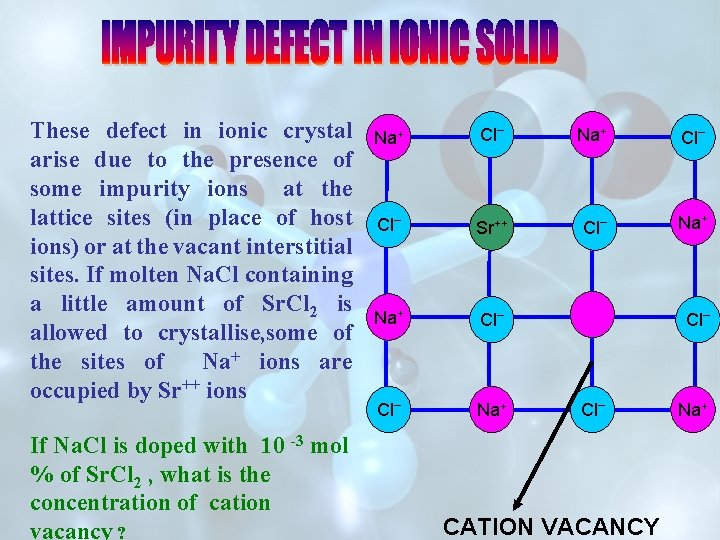
These defect in ionic crystal arise due to the presence of some impurity ions at the lattice sites (in place of host ions) or at the vacant interstitial sites. If molten Na. Cl containing a little amount of Sr. Cl 2 is allowed to crystallise, some of the sites of Na+ ions are occupied by Sr++ ions If Na. Cl is doped with 10 -3 mol % of Sr. Cl 2 , what is the concentration of cation vacancy ? Na+ Cl _ Sr++ Cl Na+ Cl _ _ Na+ Cl Cl _ CATION VACANCY _ Na+