STRUCTURE OF MATTER PRINCIPLES OF ADHESION BDS FIRST

STRUCTURE OF MATTER & PRINCIPLES OF ADHESION BDS FIRST YR DENTAL MATERIALS TIMING- 10: 00 – 11: 00 AM DATE- 15/09/2014 PRESENTED BYDR LAKSHYA KUMAR ASSISTANT PROFESSOR PROSTHODONTICS

STRUCTURE OF MATTER � Material Properties to be considered � 1. BIOCOMPATIBILITY (whether material can be used intraorally) � 2. PHYSICOCHEMICAL Properties � 3. HANDLING CHARACTERISTICS (ease of handling) � 4. AESTHETICS(relating to beauty) � 5. ECONOMY (cost effectiveness)
� � � To understand DM – Basic Knowledge of “Matter” B’coz behavior of DM – depends on atomic structure (ceramic, plasticizer, metal) Atoms & Molecules are held together by “ATOMIC INTERACTIONS”

Change of State Melting Temp. Heat of Vaporization LIQUID � SOLID GAS Latent heat of Fusion � Heat of Vaporization : when water boils ‘quantity of energy ’ needed to transform Liquid to Vapour Amount of heat needed to evaporate 1 gm of liquid to vapour (at given temp & pressure) � � Ex: - 540 cal. of heat is req. to vaporize 1 gm of H 2 O (at 100 *C & pressure of 1 atom)
KINETIC ENERGY � � Gaseous state possesses more KE than does the Liquid state If the KE of liquid decreases sufficiently when temp is decreased, 2 nd Transformation in state may occur Liquid can change to solid KE is released (in form of heat) when Liquid freezes. Latent Heat of Fusion 1 gm of H 2 O freezes, 80 cal. Of heat is released

� � 1 gm of solid is changed to liquid – input of energy is req For Metals – the temp. at which Change occurs is k/as Melting Temperature
Interatomic Bond Distance & Bonding Energy � � � Bond Distance : Limiting factor which prevents atoms / molecules from approaching each other too closely If Distance reduces – Repulsion If Distance increases – Attraction If forces of Attraction increases – Interatomic space decreases Bonding Energy: Energy can be defined as a force integrated over a distance

� � � Thermal Energy: KE of atoms/molecules at a given temp. atoms are is constant state of vibration If higher the temp. greater the amplitude so, greater is the KE/ Internal Energy. Gross effect is expansion – k/as Thermal Expansion
Crystalline Structure � � Atoms are bonded by Primary / Secondary forces, Na+ attract Cl- results in regularly spaced configuration – Space Lattice/ Crystal. Any arrangement of atoms such that every atom is situated similar to every atom
Non Crystalline Structure � � � Waxes – may solidify as amorphous materials such that the molecules are distributed at random. Glass – its atoms tends to develop a short order instead of long range order (Crystalline Structure) Ordered arrangement of glass is more/less locally interspersed with a considerable no. of disordered units because this arrangement is typical of liquids: such solids (glass)are sometimes called Supercooled Liquids
Glass Transition Temperature � The temp. at which there is an abrupt increase in thermal expansion coefficient, indicating increased molecular mobility is called Glass Transition Temperature(Tg) � It is characteristic of the particular glassy structure. � Also k/as Glass Temperature

Diffusion � � In Gases & Liquid is well-known Atoms/Molecules diffuse in solid state as well. Diffusion in Crystalline structure at room temp. is very low. At increased temp. prop. of metals may be changed radically by atomic diffusion Diffusion in Non-crystalline structure may occur at rapid rate (b. coz of disordered structure)

ADHESION & BONDING In complete denture retention – Adhesion between Denture & Saliva & Soft tissue � 2 substances brought into Intimate contact, one adhere to the other, this Force is Adhesion : In / When Unlike molecules are attracted {Cohesion: In / When Like molecules are attracted} � � � Material / film produced for Adhesion is Adhesive (fluid/semiviscous is best) Material to which it is applied is Adherend

� In Mechanical bonding there is Strong attachment, ex – Screws, Bolts, Undercuts � Internal surface of Crown / Post with cement irregularities with Air abrasion Enamel Acid Etching – Phosphoric Acid (10 -20 secs) forms minute pores – resin flows into pores � � Increased /improved Mechanical Retention – Decreased Marginal Leakage, Stains, Secondary Caries & Irritation of Pulp

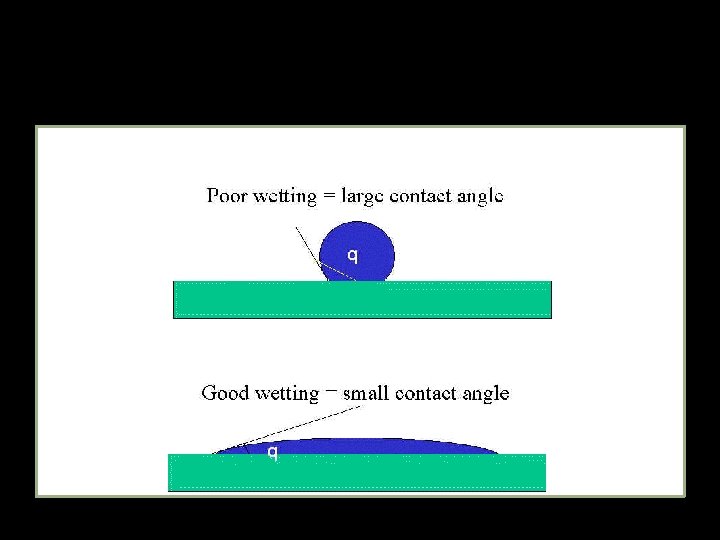
Principles of Adhesion � � � Surface Energy Wetting Contact Angle of Wetting ()

� � � Surface Energy At surface of lattice, energy is greater (outermost atoms are not equally attracted) increase in energy per unit area of surface is referred to as Surface Energy/ Tension Greater Surface energy – greater capacity of Adhesion

Principles of Adhesion � Wetting Liquid must flow easily over entire surface & adhere to solid � Contact Angle of Wetting �
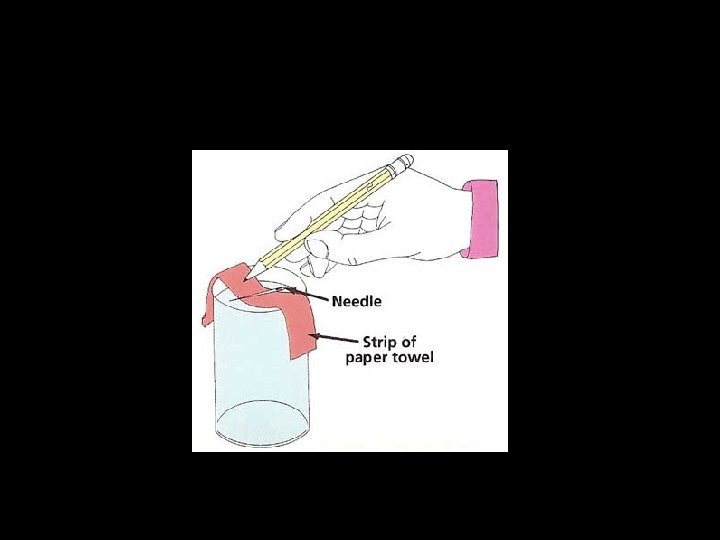

ACID ETCHING Procedure 1. Surface treatment 2. Application of acid 3. Acid concentration 4. Type of acid 5. Etching time 6. Washing stage 7. Drying stage

ACID ETCHING The pattern of Enamel etching is categorized as Type 1 (preferential prism center etching) Type 2 (preferential prism periphery etching) Type 3 (mixed). There appears to be no difference in micro-mechanical bonding of the different etching patterns. The etched surface develops a frosty appearance

ACID ETCHING Etching of dentin surfaces primarily dissolves hydroxyapatite crystals within the surface of the intertubular dentin and along the surface of the outermost peritubular dentin. A smear layer exists from cavity preparation that is typically 1 to 2 um thick with smear plugs


Interatomic Bonding: The forces that hold atoms together are called cohesive forces. These interatomic bonds may be classified as primary or secondary. The strength of these bonds and their ability to form after breakage determine the physical properties of material.
Primary Bond A bond that forms between atoms and that involves the exchanging or sharing of electrons. Secondary Bond A bond that involves attraction between molecules. Unlike primary bonding, there is no transfer or sharing of electrons.
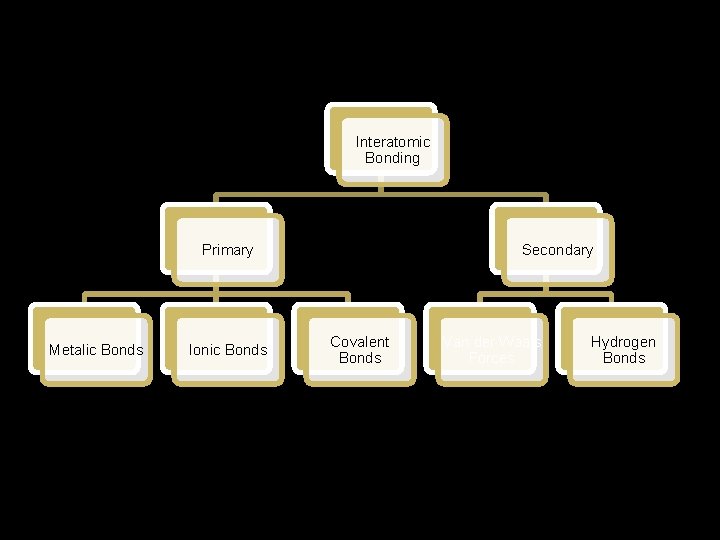
Interatomic Bonding Primary Metalic Bonds Ionic Bonds Secondary Covalent Bonds Van der Waals Forces Hydrogen Bonds
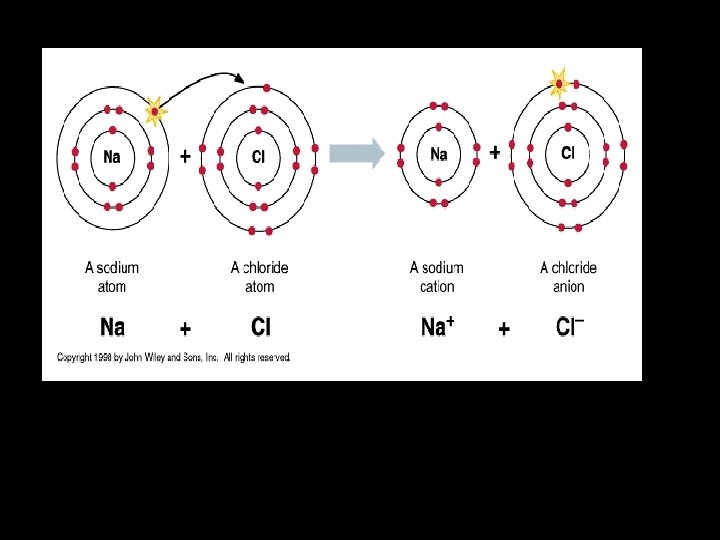
Interatomic Primary Bonding: Interatomic primary bonding may be of three different types: 1. Ionic Bonds: � � � Result from the mutual attraction of positive and negative charges. The classic example is sodium chloride (Na+Cl-). In dentistry, ionic bonding exists in certain crystalline phases of some dental materials, such as gypsum and phosphate based cement.


2. Covalent Bonds: � � � In many chemical compounds, two valence electrons shared by adjacent atoms. The hydrogen molecule H 2, is an example of covalent bonding. Covalent bonding occur in many organic compounds, such as dental resin.


3. Metallic Bonds: � � � It is the attraction force between positive metal ions and the delocalized (freely moving) electrons, gathered in an electron cloud. These free electrons are responsible for the high electric and thermal conductivities of metals also for their ability to deform plastically. Found only in metals.

Interatomic secondary Bonding: In contrast with primary bonds, secondary bonds don’t share electrons. Instead, charge variations among molecules or atomic groups induce polar forces that attract the molecules.

1. Hydrogen Bonding: � � Bonds between hydrogen atom and atoms of the most electronegative elements (N, O, F) are called hydrogen bonds. When a water molecule intermingle with other water molecules, the hydrogen (positive) portion of one molecule is attached to the oxygen (negative) portion of its neighboring molecule and hydrogen bridges are formed. Polarity of this nature is important in accounting for the intermolecular reaction in many organic compounds, such as the absorption of water by synthetic dental resins.


2. Van der Waals Forces: � � Van der Waals Forces form the basis of a dipole attraction. Normally, the electrons of the atoms are distributed equally around the nucleus and produce an electrostatic field around the atom. However this field may fluctuate so that its charge becomes momentarily positive and negative. A fluctuating dipole is thus created that will attract other similar dipoles. Such interatomic forces are quite weak.


� In general, materials can be subdivided into two categories according to their atomic arrangement. In crystalline materials there is a three-dimensional periodic pattern of the atoms, whereas no such long range periodicity is present in noncrystalline materials, which possess only short-range atomic order.

Crystalline Structure: � Atoms are bonded to each other by either primary or secondary forces. In the solid state, they combine in a manner that ensures minimal internal energy. The result is that they form a regularly spaced configuration known as a space lattice or crystal.

� A space lattice can be defined as any arrangement of atom in space in which every atom is situated similarly to every other atom. Space lattices may be the result of primary or secondary bonds

� There are 14 possible lattice types or forms, but many of the metals used in dentistry belong to the cubic system; that is, the atoms crystallize in cubic arrangements. All dental amalgams, cast alloys, wrought metals, gold foil are crystalline. Some pure ceramics, such as aluminia and zirconia core ceramics, are entirely crystalline

� Other ceramics, such as porcelains, consists of noncrystalline glass matrix and crystalline inclusions that provide desired properties, including color, opacity, and increase in thermal expansion coefficients, radiopacity, strength, fracture toughness.

Simple cubic

Body centered cubic � � In the body-centered cubic (BCC) array, All angles are 90 degrees and all atoms are equidistant from one another in the horizontal and vertical directions. Metallic atoms are located at the corners of the unit cell, and one atom is at the center of the unit cell
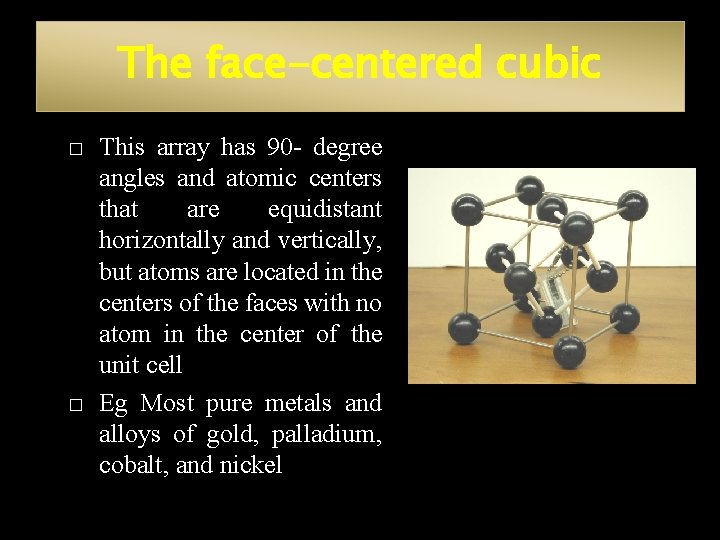
The face-centered cubic � � This array has 90 - degree angles and atomic centers that are equidistant horizontally and vertically, but atoms are located in the centers of the faces with no atom in the center of the unit cell Eg Most pure metals and alloys of gold, palladium, cobalt, and nickel

Face centered orthorhombic
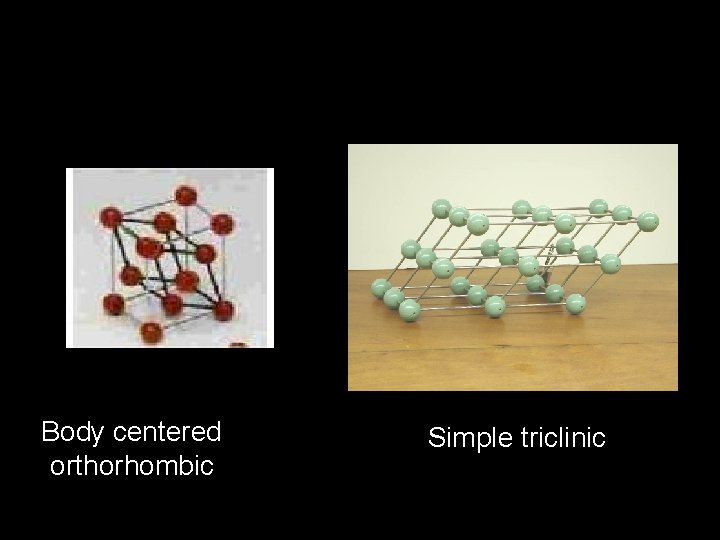
Body centered orthorhombic Simple triclinic
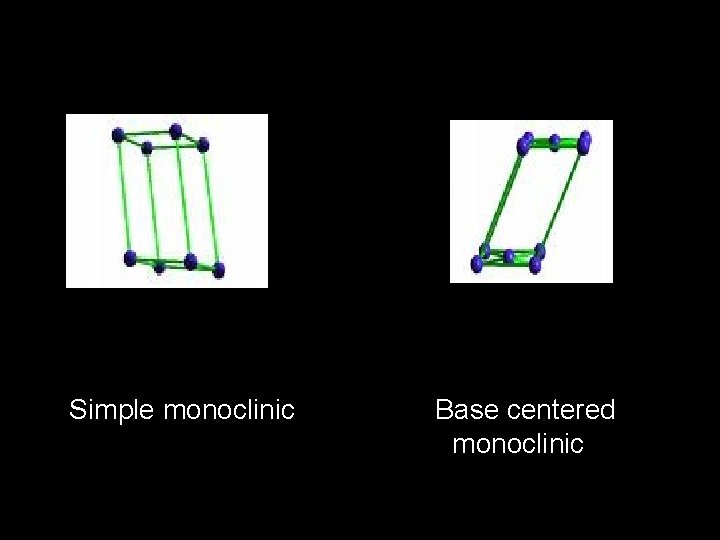
Simple monoclinic Base centered monoclinic

Noncrystalline Solids and their Structures: � � Structures other than crystalline forms can occur in the solid state. For example, waxes may solidify as amorphous materials so that the molecules are distributed at random. . A resin based composite consists of resin matrix, filler particles and an organic coupling agent that bond the filler particles to the resin matrix. In some cases, the filler particles are made from radiopaque glasses that are nancrystalline.

� � Composites have a noncrystalline matrix and may or may not contain crystalline filler particles. The structural arrangements of the noncrystalline solids don’t represent such low internal energies as do crystalline arrangements of the same atoms and molecules. Noncrystalline solids do not have a definite melting temperature, but rather they gradually soften as the temperature is raised.

THANK YOU
- Slides: 54