Structure of Matter From Biophysical Point of View

Structure of Matter From Biophysical Point of View Assoc. Prof. RNDr. Mgr. Katarína Kozlíková, CSc. IMPh. BPh. ITM FM CU in Bratislava katarina. kozlikova@fmed. uniba. sk The presentation was originally created as a part of the project KEGA 004 UK-4/2011 (MESR&S SR): „Electromagnetic biosignals and electromagnetic radiation – electronic education of Medical Biophysics (creation of e-learning courses)“ Principal investigator: Assoc. Prof. RNDr. Mgr. Katarína Kozlíková, CSc.

Contents n n n Fundamental problem of science Important stages in structure analysis Structure of matter Forms of matter Physical interactions General properties of matter General characteristics of matter Heisenberg uncertainty principle Elementary particles Schrödinger equation - principle Appendix © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 2
Fundamental Problem of Science n n Relation between the structure of matter and its function Methods of structure analysis – Better understanding of the functioning of living organisms n n Considerably extended by use of microscopes Biological studies could be extended to – Molecular levels – Atomic levels n New methods and a deeper insight call for new disciplines – Emergence of a new branch of science connected with n n © K. Kozlíková, 2019 A new approach to reasoning A new scientific attitude Structure of Matter From Biophysicsl Point of View 3
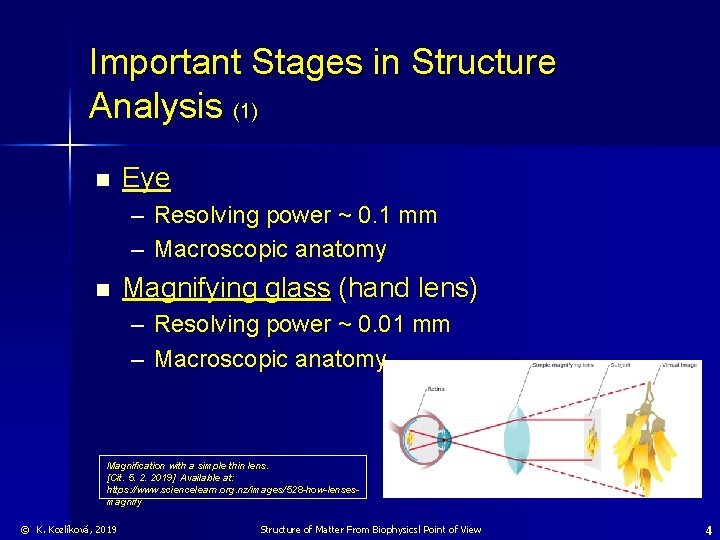
Important Stages in Structure Analysis (1) n Eye – Resolving power ~ 0. 1 mm – Macroscopic anatomy n Magnifying glass (hand lens) – Resolving power ~ 0. 01 mm – Macroscopic anatomy Magnification with a simple thin lens. [Cit. 5. 2. 2019] Available at: https: //www. sciencelearn. org. nz/images/528 -how-lensesmagnify © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 4
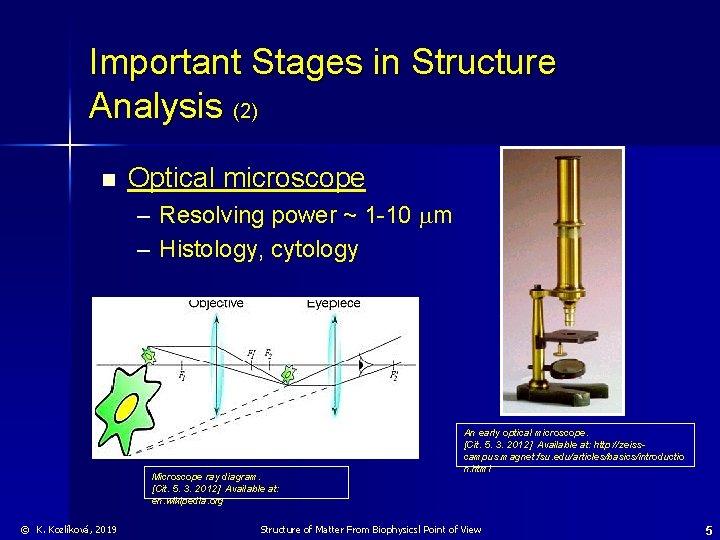
Important Stages in Structure Analysis (2) n Optical microscope – Resolving power ~ 1 -10 m – Histology, cytology Microscope ray diagram. [Cit. 5. 3. 2012] Available at: en. wikipedia. org © K. Kozlíková, 2019 An early optical microscope. [Cit. 5. 3. 2012] Available at: http: //zeisscampus. magnet. fsu. edu/articles/basics/introductio n. html Structure of Matter From Biophysicsl Point of View 5
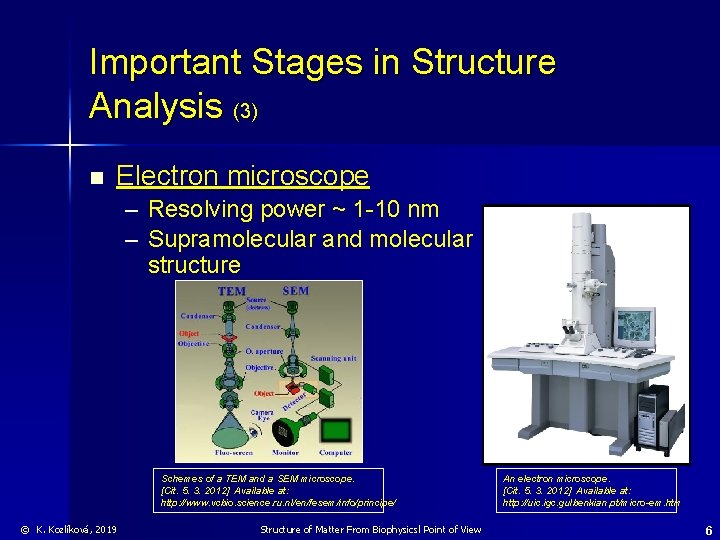
Important Stages in Structure Analysis (3) n Electron microscope – Resolving power ~ 1 -10 nm – Supramolecular and molecular structure Schemes of a TEM and a SEM microscope. [Cit. 5. 3. 2012] Available at: http: //www. vcbio. science. ru. nl/en/fesem/info/principe/ © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View An electron microscope. [Cit. 5. 3. 2012] Available at: http: //uic. igc. gulbenkian. pt/micro-em. htm 6
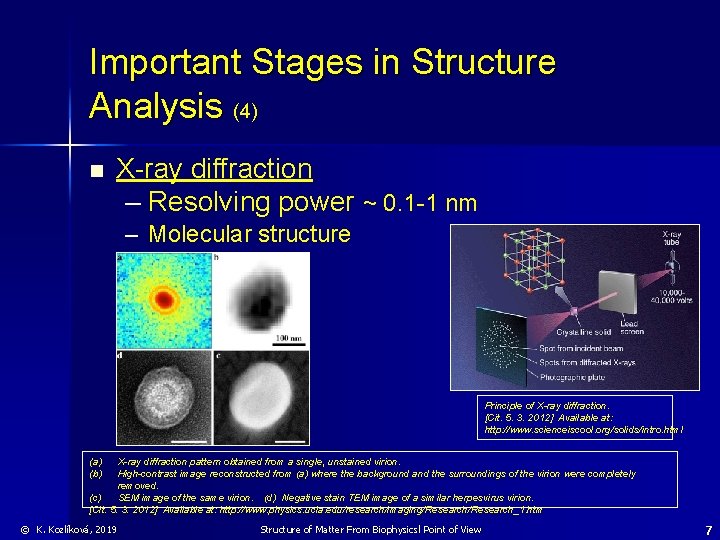
Important Stages in Structure Analysis (4) n X-ray diffraction – Resolving power ~ 0. 1 -1 nm – Molecular structure Principle of X-ray diffraction. [Cit. 5. 3. 2012] Available at: http: //www. scienceiscool. org/solids/intro. html (a) (b) X-ray diffraction pattern obtained from a single, unstained virion. High-contrast image reconstructed from (a) where the background and the surroundings of the virion were completely removed. (c) SEM image of the same virion. (d) Negative stain TEM image of a similar herpesvirus virion. [Cit. 5. 3. 2012] Available at: http: //www. physics. ucla. edu/research/imaging/Research_1. htm © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 7
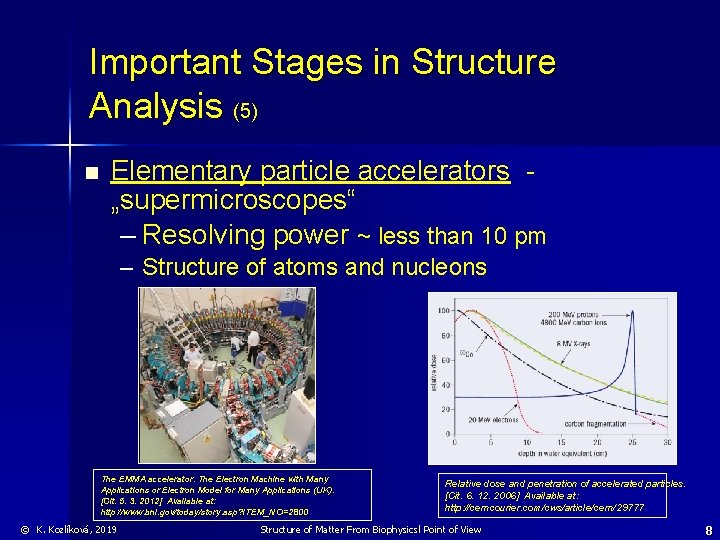
Important Stages in Structure Analysis (5) n Elementary particle accelerators „supermicroscopes“ – Resolving power ~ less than 10 pm – Structure of atoms and nucleons The EMMA accelerator. The Electron Machine with Many Applications or Electron Model for Many Applications (UK). [Cit. 5. 3. 2012] Available at: http: //www. bnl. gov/today/story. asp? ITEM_NO=2800 © K. Kozlíková, 2019 Relative dose and penetration of accelerated particles. [Cit. 6. 12. 2006] Available at: http: //cerncourier. com/cws/article/cern/29777 Structure of Matter From Biophysicsl Point of View 8
Structure of Matter n n n Every body – living or inanimate – is built up from elements – atoms Molecules, their systems, organisation within these are determined by atomic and molecular interactions Various systems display – General properties of matter – Special properties due to their composition and organisation n Special properties account for the phenomenon called life © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 9

Two Basic Forms of Matter n Corpuscular – Built up by particles with rest mass n Force fields – Represented by physical interactions among particles (quanta of fields) n n They are of equal importance and complement each other Wave particle – Term expressing the basic property that matter is both a particle and a field n Quantum theory (quantum physics) – Describes the behaviour of the smallest particles of matter © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 10
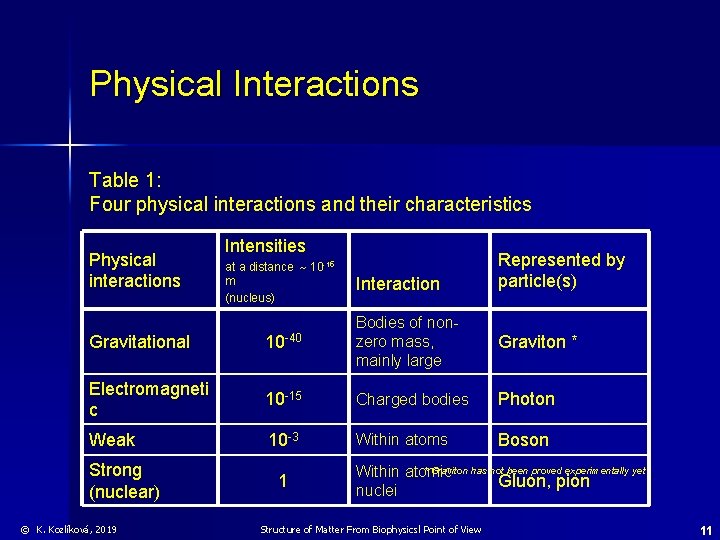
Physical Interactions Table 1: Four physical interactions and their characteristics Physical interactions Intensities at a distance 10 -15 m (nucleus) Interaction Represented by particle(s) Gravitational 10 -40 Bodies of nonzero mass, mainly large Electromagneti c 10 -15 Charged bodies Photon Weak 10 -3 Within atoms Boson Strong (nuclear) © K. Kozlíková, 2019 1 Graviton * * Graviton has not been proved experimentally yet Within atomic Gluon, pion nuclei Structure of Matter From Biophysicsl Point of View 11
Physical Fields May Display Corpuscular Properties - Example n Light can behave as – An electromagnetic wave n Several phenomena – A corpuscle (a discrete particle) n n Interaction with atoms and molecules A light quantum – a photon – a particle moving with velocity c is characterised by – – – © K. Kozlíková, 2019 Energy E Mass m Momentum p Frequency f Wavelength Structure of Matter From Biophysicsl Point of View 12
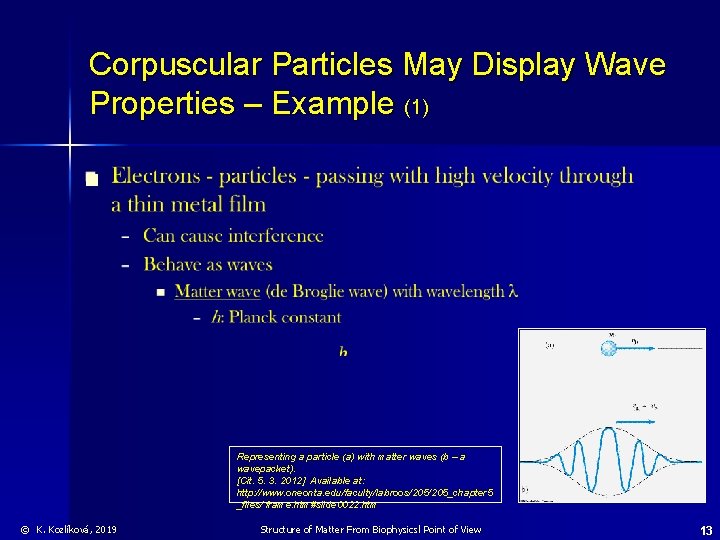
Corpuscular Particles May Display Wave Properties – Example (1) n Representing a particle (a) with matter waves (b – a wavepacket). [Cit. 5. 3. 2012] Available at: http: //www. oneonta. edu/faculty/labroos/205_chapter 5 _files/ frame. htm#slide 0022. htm © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 13
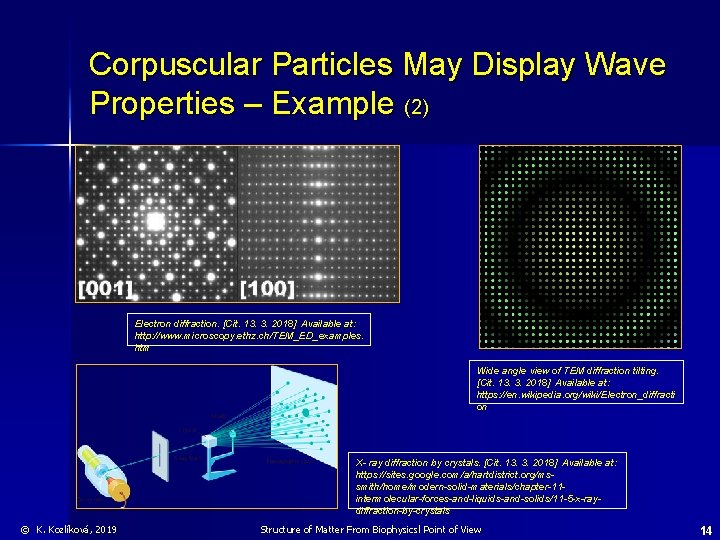
Corpuscular Particles May Display Wave Properties – Example (2) Electron diffraction. [Cit. 13. 3. 2018] Available at: http: //www. microscopy. ethz. ch/TEM_ED_examples. htm Wide angle view of TEM diffraction tilting. [Cit. 13. 3. 2018] Available at: https: //en. wikipedia. org/wiki/Electron_diffracti on X- ray diffraction by crystals. [Cit. 13. 3. 2018] Available at: https: //sites. google. com/a/hartdistrict. org/mssmith/home/modern-solid-materials/chapter-11 intermolecular-forces-and-liquids-and-solids/11 -5 -x-raydiffraction-by-crystals © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 14
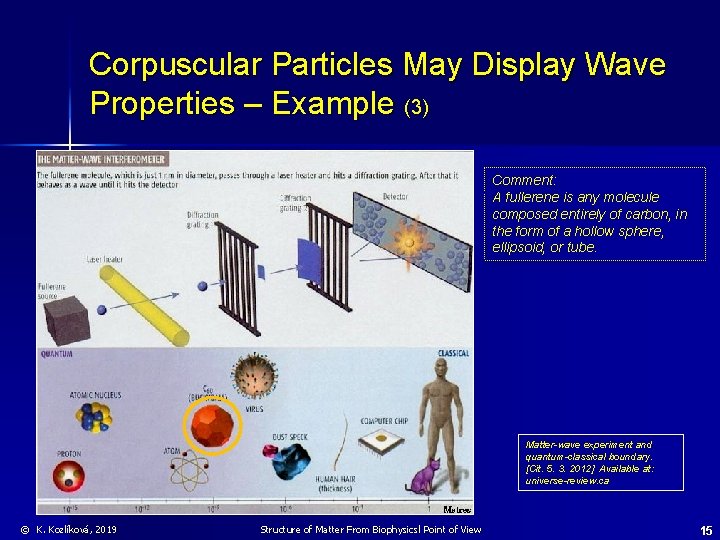
Corpuscular Particles May Display Wave Properties – Example (3) Comment: A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Matter-wave experiment and quantum-classical boundary. [Cit. 5. 3. 2012] Available at: universe-review. ca © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 15
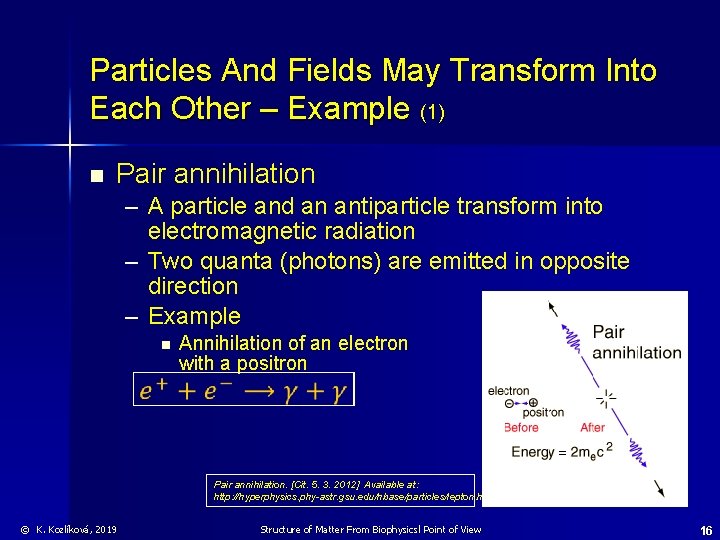
Particles And Fields May Transform Into Each Other – Example (1) n Pair annihilation – A particle and an antiparticle transform into electromagnetic radiation – Two quanta (photons) are emitted in opposite direction – Example n Annihilation of an electron with a positron Pair annihilation. [Cit. 5. 3. 2012] Available at: http: //hyperphysics. phy-astr. gsu. edu/hbase/particles/lepton. html © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 16
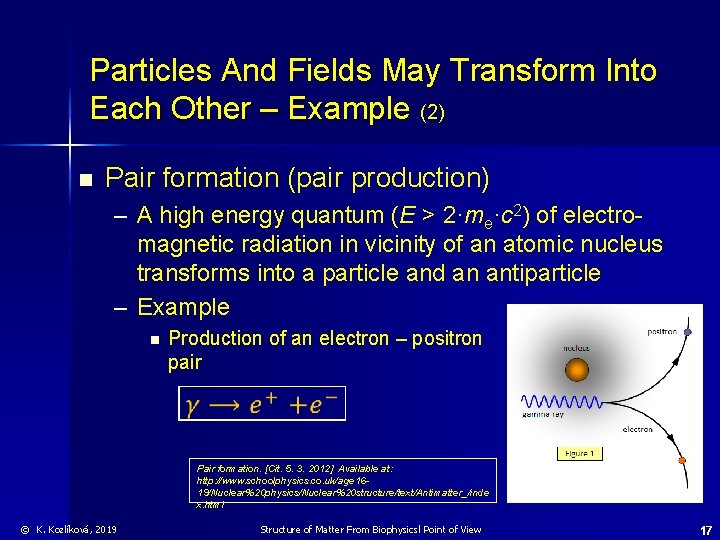
Particles And Fields May Transform Into Each Other – Example (2) n Pair formation (pair production) – A high energy quantum (E > 2·me·c 2) of electromagnetic radiation in vicinity of an atomic nucleus transforms into a particle and an antiparticle – Example n Production of an electron – positron pair Pair formation. [Cit. 5. 3. 2012] Available at: http: //www. schoolphysics. co. uk/age 1619/Nuclear%20 physics/Nuclear%20 structure/text/Antimatter_/inde x. html © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 17
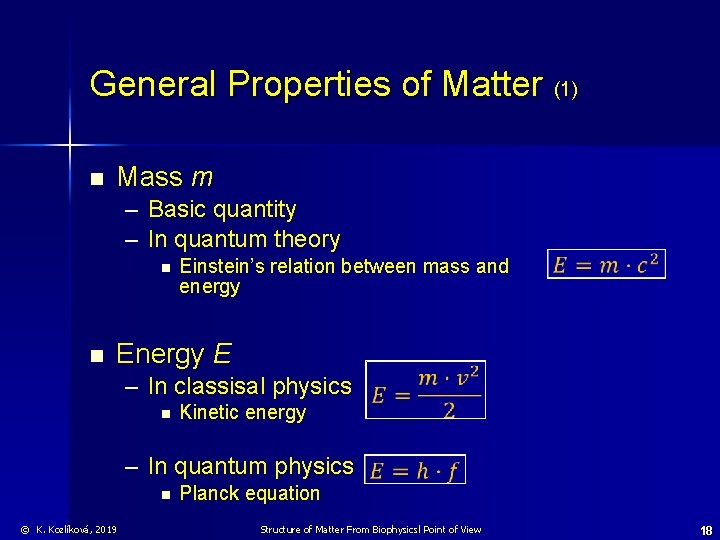
General Properties of Matter (1) n Mass m – Basic quantity – In quantum theory n n Einstein’s relation between mass and energy E – In classisal physics n Kinetic energy – In quantum physics n © K. Kozlíková, 2019 Planck equation Structure of Matter From Biophysicsl Point of View 18
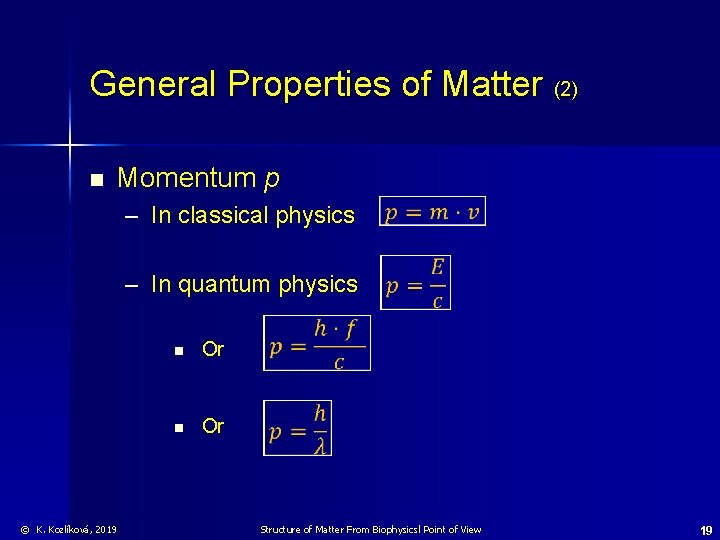
General Properties of Matter (2) n Momentum p – In classical physics – In quantum physics © K. Kozlíková, 2019 n Or Structure of Matter From Biophysicsl Point of View 19
General Properties of Matter (3) n Frequency f – In classical physics (T is period) – In quantum physics n Wavelength A wave diagram. [Cit. 5. 3. 2012] Available at: http: //www. bbc. co. uk/scotland/learning/bitesize/hi gher/physics/radiation/waves_rev 1. shtml © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 20

General Characteristics of Matter n Matter always has – Mass m – Energy E n Matter may have – Momentum p – Angular momentum L n During interactions or transformations – Exchange of mass and energy may occur – Conservation laws of mass, energy, momentum, angular momentum, electric charge remain valid © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 21
Heisenberg Uncertainty Principle (1) n In quantum mechanics, this principle states a fundamental limit on the accuracy, with which certain pairs of physical properties can be simultaneously known, for example – Position and momentum – Energy and time n The uncertainty principle states a fundamental property of quantum systems, and is not a statement about the observational success of current technology – Every measurement destroys a part of our knowledge of the system that was obtained by previous measurements © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 22
Heisenberg Uncertainty Principle (2) n Position and momentum – Precise knowledge of the position of a particle (the uncertainty of the position is zero: x = 0) rules out obtaining any information concerning the momentum of the particle (the uncertainty of the momentum is infinitely large: p ) n Energy and time – If we exactly know the energy released or absorbed by a particle, for example accompanying the jump of an electron from energy level to another (the uncertainty of the energy is zero: E = 0), we know nothing of the time needed to execute this process (the uncertainty of the time is infinitely large: t ) © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 23
Elementary Particles (1) n n Fundamental particles Any of the subatomic particles that compose matter and energy, especially one hypothesized or regarded as an irreducible constituent of matter Each elementary particle is characterised by rest mass, rest energy, electric charge, life time, spin, and other quantum numbers Occur in pairs of a particle and an antiparticle – Their characteristics are identical in value, but opposite in sign © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 2019 24
Elementary Particles (2) n n More than 200 available (in broader sense) Elementary particles (in narrower sense) – Elementary fermions (matter particles) n n 6 leptons (electron, mion, tauon and their neutrinos) 6 quarks (up, down, charm, strange, top, bottom) – Elementary bosons (force-carrying particles, associated with fundamental interactions) n n n Gauge bosons (photon, gluon, W boson, Z boson) Higgs boson For medical purposes needed – Electron and positron n Electron neutrino and antineutrino – Proton and neutron – Photon © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View Proton quark structure. [Cit. 18. 4. 2012] Available at: http: //en. wikipedia. org/wiki/List_of_particles 25
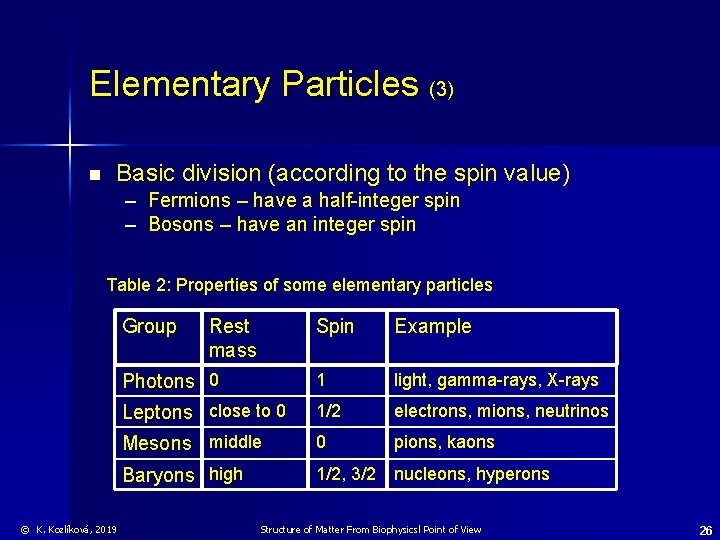
Elementary Particles (3) n Basic division (according to the spin value) – Fermions – have a half-integer spin – Bosons – have an integer spin Table 2: Properties of some elementary particles Group © K. Kozlíková, 2019 Rest mass Spin Example Photons 0 1 light, gamma-rays, X-rays Leptons close to 0 1/2 electrons, mions, neutrinos Mesons middle 0 pions, kaons Baryons high 1/2, 3/2 nucleons, hyperons Structure of Matter From Biophysicsl Point of View 26
Schrödinger Equation (1) n All properties of elementary particles and their sets can be quantitatively expressed by Schrödinger equation – It has the same position in quantum mechanics as the Newton’s laws in classical mechanics – Mathematical expression needs differential calculus © K. Kozlíková, 2019 A harmonic oscillator in classical mechanics (A-B) and quantum mechanics (C-H). [Cit. 5. 3. 2012] Available at: http: //en. wikipedia. org/wiki/Schr%C 3%B 6 dinger_equat ion Structure of Matter From Biophysicsl Point of View 27
Schrödinger Equation (2) n Solution of the equation is the wave function – Includes spatial co-ordinates, energy, time n The squared absolute value of the wave function ² expresses the probability density of a particle occurrence – Only some values of energies can be the solutions of the equation n n There is no continual spectrum of energies The coefficients determining the possible energy states are called quantum numbers © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 28
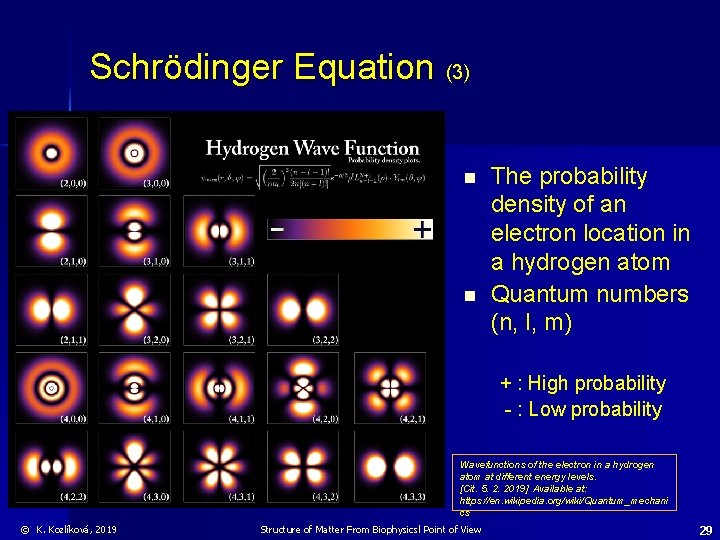
Schrödinger Equation (3) n n The probability density of an electron location in a hydrogen atom Quantum numbers (n, l, m) + : High probability - : Low probability Wavefunctions of the electron in a hydrogen atom at different energy levels. [Cit. 5. 2. 2019] Available at: https: //en. wikipedia. org/wiki/Quantum_mechani cs © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 29

Literature n HRAZDIRA, I. , MORNSTEIN, V. , BOUREK, A. , ŠKORPÍKOVÁ, J. Fundamentals of Biophysics and Medical Technology. Brno : Masaryk University, Faculty of Medicine, 2007. 325 p. ISBN 978 -80 -210 -4228 -5. n KOZLÍKOVÁ, K. , MARTINKA, J. Theory And Tasks For Practicals On Medical Biophysics. Brno : Librix, 2010. 248 p. ISBN 978 -80 -7399 -881 -3 n RONTÓ, G. , TARJÁN, I. (eds. ) An Introduction To Biophysics With Medical Orientation. Budapest : Akadémiai Kiadó, 1997. 447 p. ISBN 963 -05 -7607 -4. n Wikipedia. [accessed repeatedly from September 2011 till February 2019] Available at: http: //en. wikipedia. org/. n Electronic sources listed directly in the text. © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 30
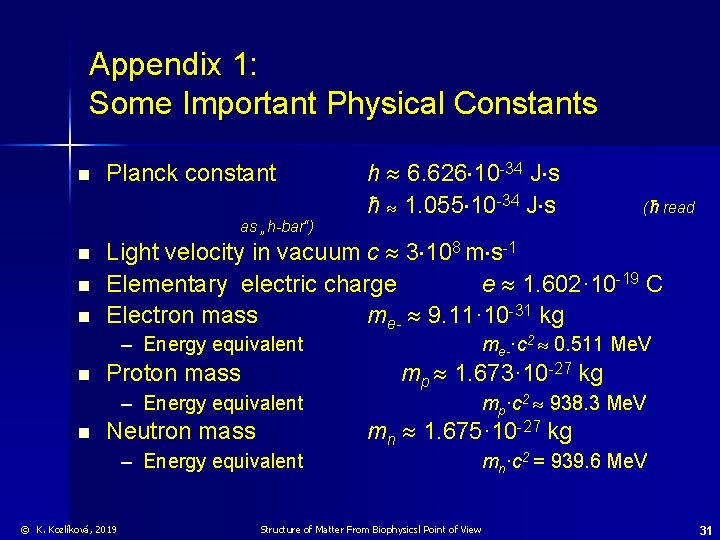
Appendix 1: Some Important Physical Constants n Planck constant as „h-bar“) n n n mp·c 2 938. 3 Me. V mn 1. 675· 10 -27 kg Neutron mass – Energy equivalent © K. Kozlíková, 2019 me-·c 2 0. 511 Me. V mp 1. 673· 10 -27 kg Proton mass – Energy equivalent n (ħ read Light velocity in vacuum c 3 108 m s-1 Elementary electric charge e 1. 602· 10 -19 C Electron mass me- 9. 11· 10 -31 kg – Energy equivalent n h 6. 626 10 -34 J s ħ 1. 055 10 -34 J s mn·c 2 = 939. 6 Me. V Structure of Matter From Biophysicsl Point of View 31

Appendix 2: Annihilation in General n Annihilation – Occurs when a particle collides with an antiparticle – It transforms mass into energy of some form – The energy is carried by force carriers particles - that can decay into other particles – It means that, contrary to a popular belief, the annihilation produces more than just two photons – What is produced, depends on the particles and antiparticles involved © K. Kozlíková, 2019 Structure of Matter From Biophysicsl Point of View 32
- Slides: 32