Structure of Materials 1 The Structure of An










- Slides: 23

Structure of Materials 1

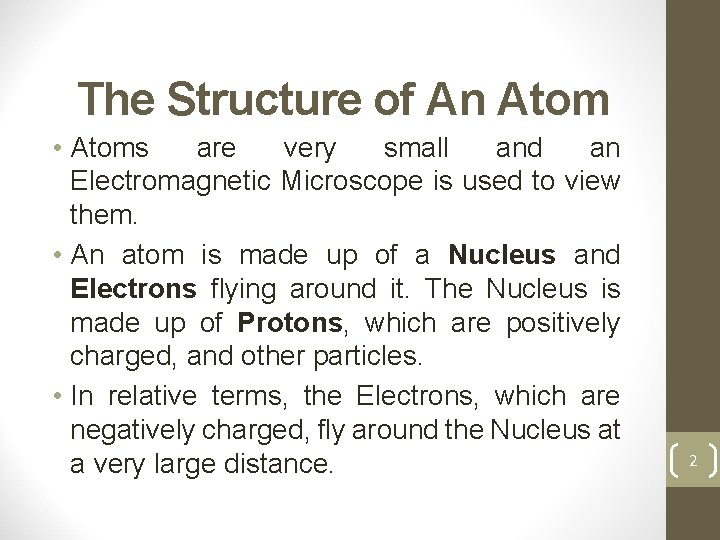
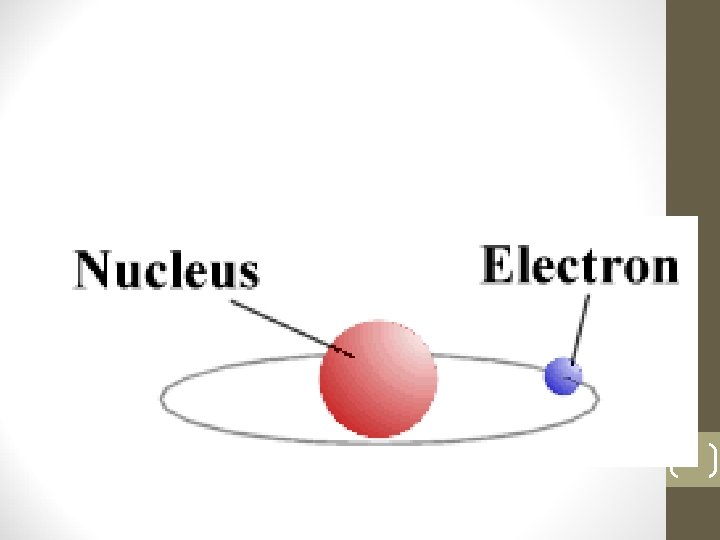
The Structure of An Atom • Atoms are very small and an Electromagnetic Microscope is used to view them. • An atom is made up of a Nucleus and Electrons flying around it. The Nucleus is made up of Protons, which are positively charged, and other particles. • In relative terms, the Electrons, which are negatively charged, fly around the Nucleus at a very large distance. 2

3

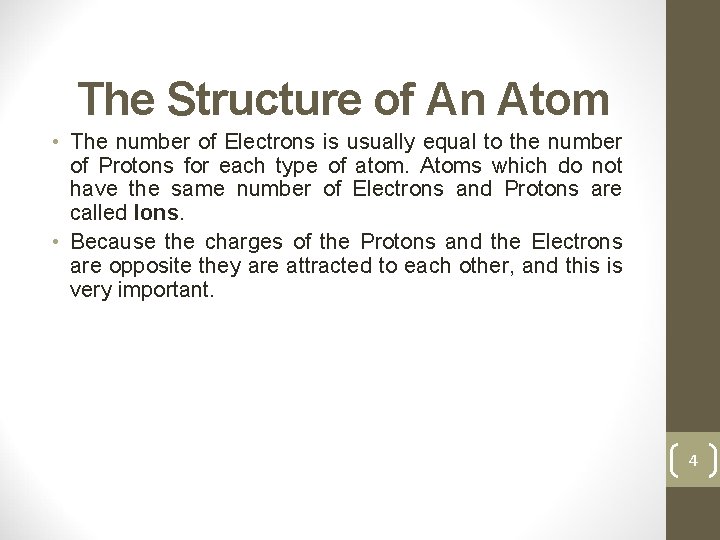
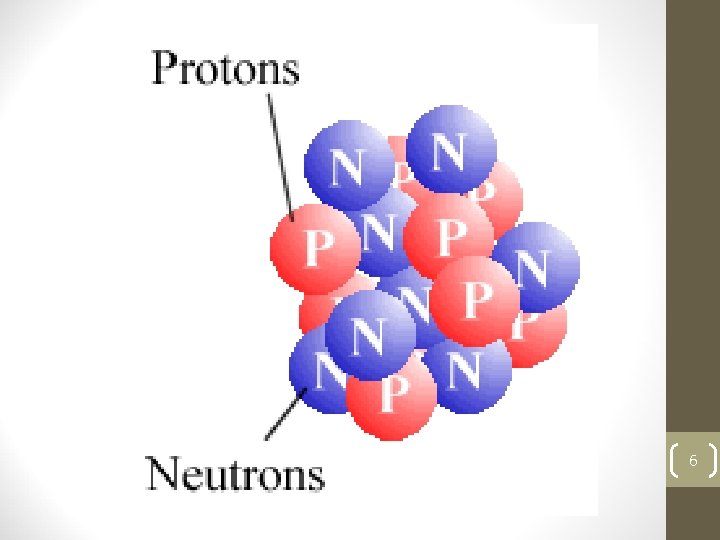
The Structure of An Atom • The number of Electrons is usually equal to the number of Protons for each type of atom. Atoms which do not have the same number of Electrons and Protons are called Ions. • Because the charges of the Protons and the Electrons are opposite they are attracted to each other, and this is very important. 4

Electrostatic Forces • Electrostatic Forces work in much the same way as magnetic forces. Like forces repel and unlike forces attract. • Electrostatic Induction is what occurs in cases such as when you rub a balloon and then it can stick to your jumper. • What happens is that the negative charge on the balloon, caused by the rubbing, repels the negative charges in your jumper causing the positive charges to be attracted to the balloon. Thus they stick. 5

6

How Atoms Bond Together • Atoms generally bond together in 3 different ways. They are : • Metallic Bonding • Covalent Bonding • Ionic Bonding 7

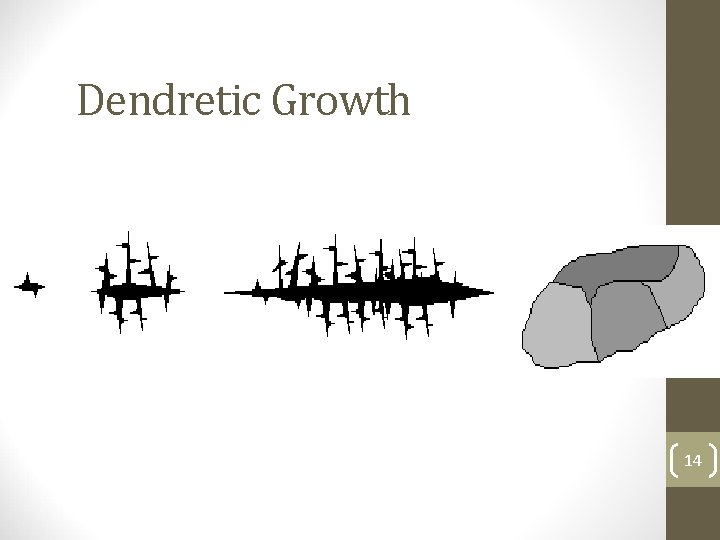
Solidifying Metal And Dendretic Growth • The cooling of a metal from a liquid to a solid is extremely important. As a metal reaches its cooling point small particles cool first. Solidification takes place in a pattern. • This pattern is called Dendretic Growth and looks like the branches of a tree. Each small particle grows to form a crystal or grain. Crystals grow together to form a solid. • The solidification starts at one point and spreads out like the branches of a tree. Eventually the Dendrite branches meet and form grains as in the final diagram. 8

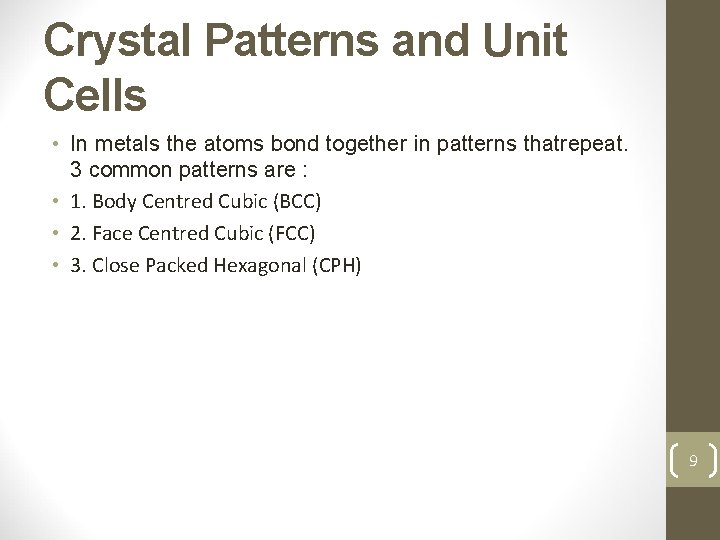
Crystal Patterns and Unit Cells • In metals the atoms bond together in patterns thatrepeat. 3 common patterns are : • 1. Body Centred Cubic (BCC) • 2. Face Centred Cubic (FCC) • 3. Close Packed Hexagonal (CPH) 9

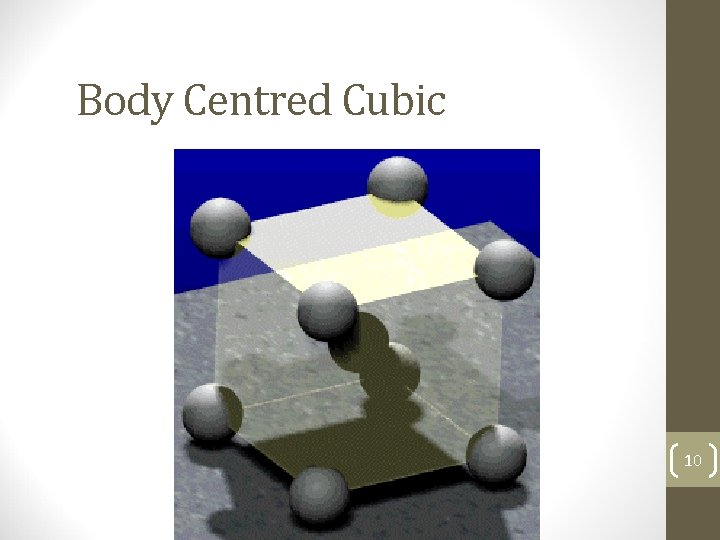
Body Centred Cubic 10

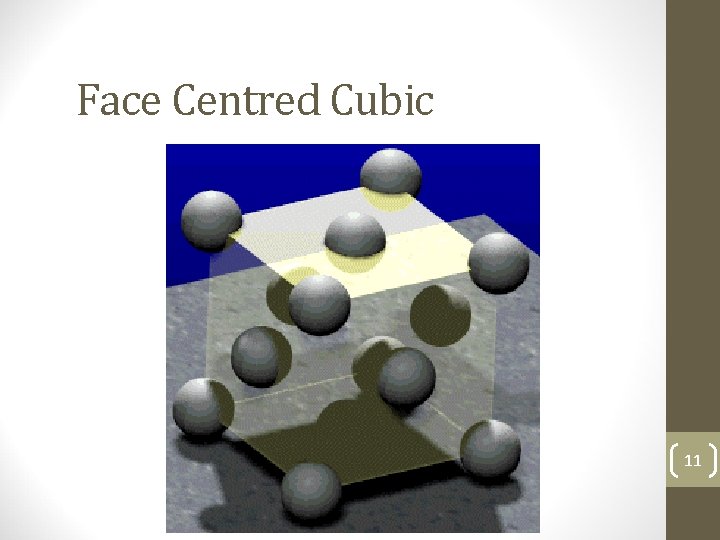
Face Centred Cubic 11

Close Packed Hexagonal 12

• A single pattern is called a Unit Cell. A group of patterns is called a Lattice. • The Unit Cells join together in three dimensions to form the Lattice, through Dendritic Growth, finally resulting in a Crystal or Grain. The formation of the grain follows the sequence shown below. 13

Dendretic Growth 14

Slip In BCC & FCC Metals • Atoms in a BCC structure are not closely packed and so a large force is required to cause them to slip. As a result shearing is less likely to occur. Therefore brittle metals have a BCC atomic structure. • Atoms in a FCC structure are more closely packed together. Therefore slip occurs more easily. Therefore shear is more likely to occur. 15

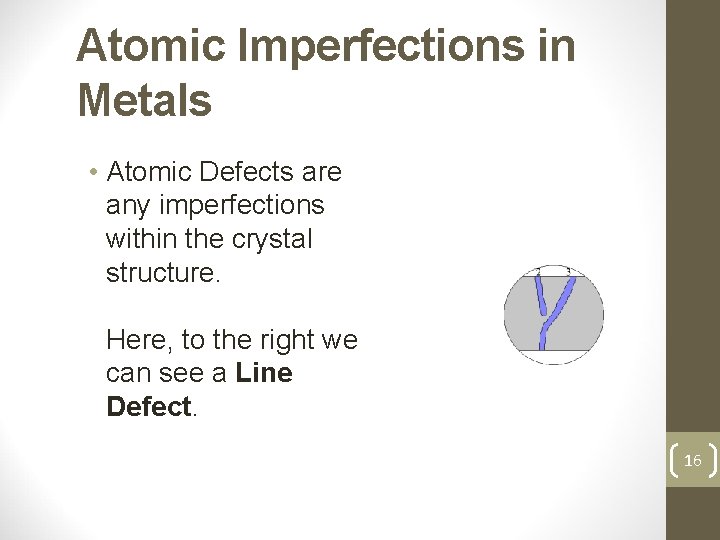
Atomic Imperfections in Metals • Atomic Defects are any imperfections within the crystal structure. Here, to the right we can see a Line Defect. 16

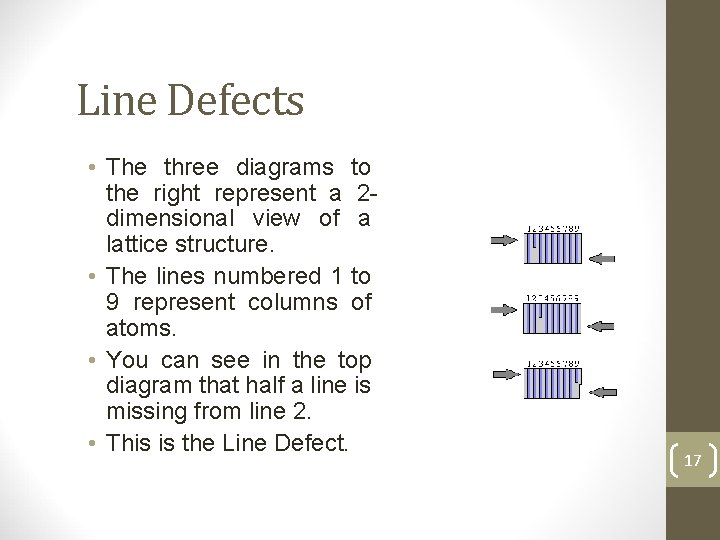
Line Defects • The three diagrams to the right represent a 2 dimensional view of a lattice structure. • The lines numbered 1 to 9 represent columns of atoms. • You can see in the top diagram that half a line is missing from line 2. • This is the Line Defect. 17

• When a Shearing force, (represented by the arrows), is applied the Line Defect 'moves' along to the next line, as you can see below. • This defect continues to move along the columns of atoms, until it reaches the end of the material, or the shearing force stops. • Because the crystal structure of metals is not perfect slip occurs. This defect gives metals their Ductile property. 18

• 1. 2. 3. Another type of defect commonly found in metal crystaline structures is called a Point Defect. There are three basic types Vacancy Substitutional Interstitial 19

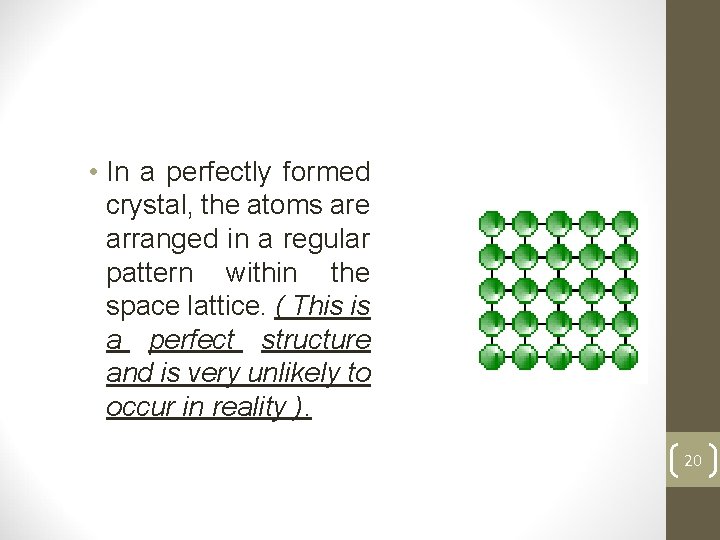
• In a perfectly formed crystal, the atoms are arranged in a regular pattern within the space lattice. ( This is a perfect structure and is very unlikely to occur in reality ). 20

Vacant Site • If there is an atom missing from the lattice, then the whole lattice is distorted as the others atoms are forced into the vacant space. This is known as a Vacant Site Defect. 21

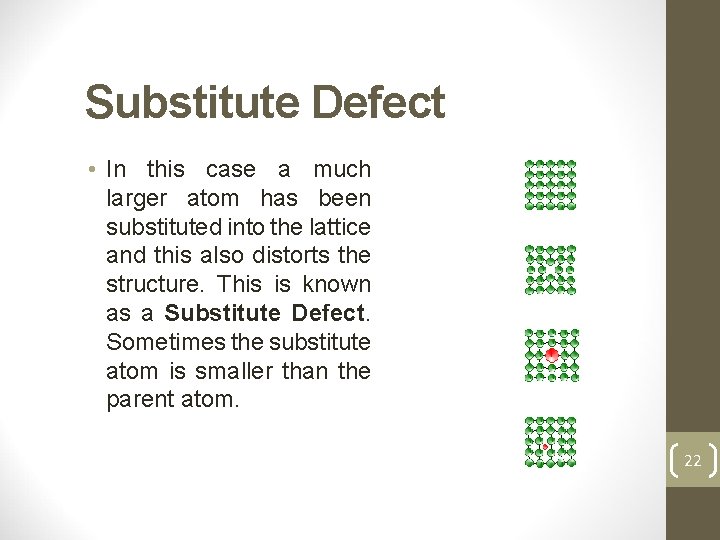
Substitute Defect • In this case a much larger atom has been substituted into the lattice and this also distorts the structure. This is known as a Substitute Defect. Sometimes the substitute atom is smaller than the parent atom. 22

Interstitial Crystal Defect • This diagram shows an Interstitial Crystal Defect where a foreign atom has moved into the space between the atoms of the lattice. Again the substitute atom can be larger than the parent atoms. 23