Structure of Diborane and Higher Boranes by M

Structure of Diborane and Higher Boranes by M GOPI Lecturer in chemistry Dr V S K Govt. Degree college(A) Visakhapatnam Andhra Pradesh.

In Diborane, the Central Atom. Boron Atomic Number-5 Electronic Configuration in ground state 1 s 2 2 px 12 py 02 pz 0 Electronic Configuration in 1 st Excited State- 1 s 2 2 s 1 2 px 12 py 12 pz 0
Di Borane (B 2 H 6) and Ethane (C 2 H 6) are similar in molecular formula. If we consider Diborane, there are 12 valence electrons for chemical bonding formation(B has 3, and H has 1, so 2 B + 6 x. H =12) and in the same way Ethane has 14 valency electrons. So Ethane can have seven Covalent bonds structure but diborane canot have the same structure due to only 12 valency electrons.
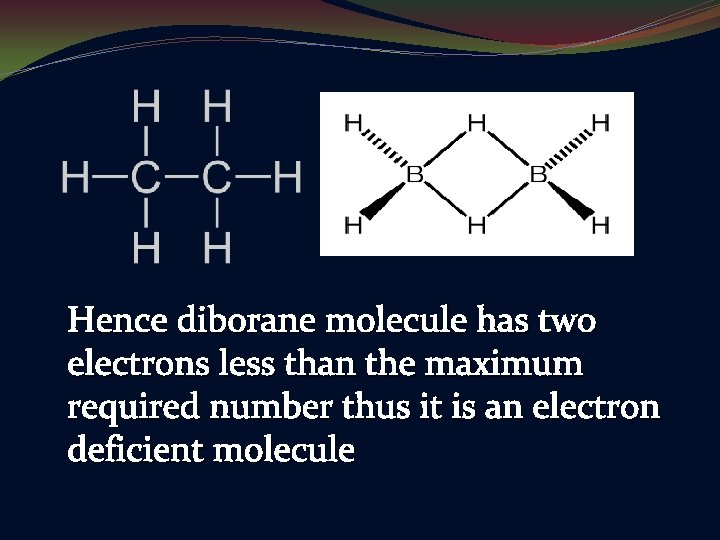
Hence diborane molecule has two electrons less than the maximum required number thus it is an electron deficient molecule

In Diborane, The Boron under goes SP 3 Hybridization due to mixing of valency S and P orbitals of Boron. So, each boron has four 3 SP Hybrid Orbitals in Which, Three are having single electrons and one is vacant.
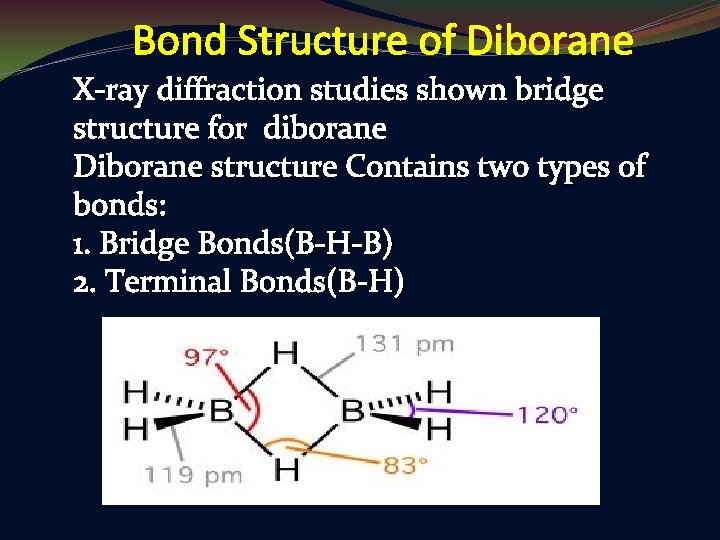
Bond Structure of Diborane X-ray diffraction studies shown bridge structure for diborane Diborane structure Contains two types of bonds: 1. Bridge Bonds(B-H-B) 2. Terminal Bonds(B-H)
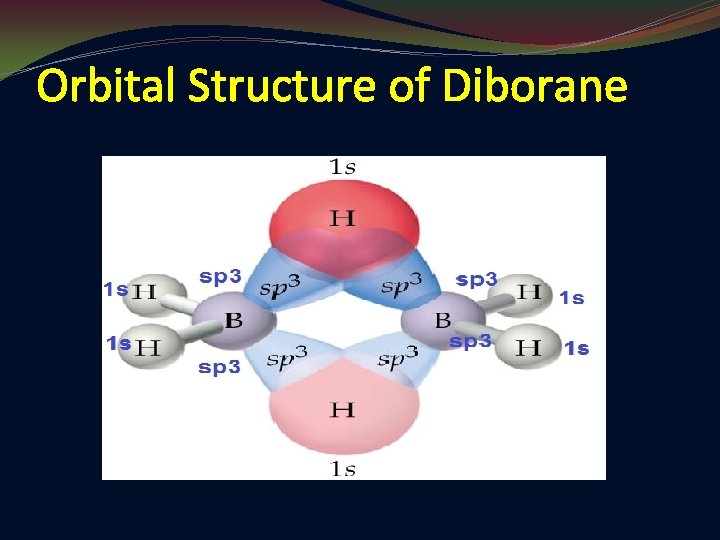
Orbital Structure of Diborane
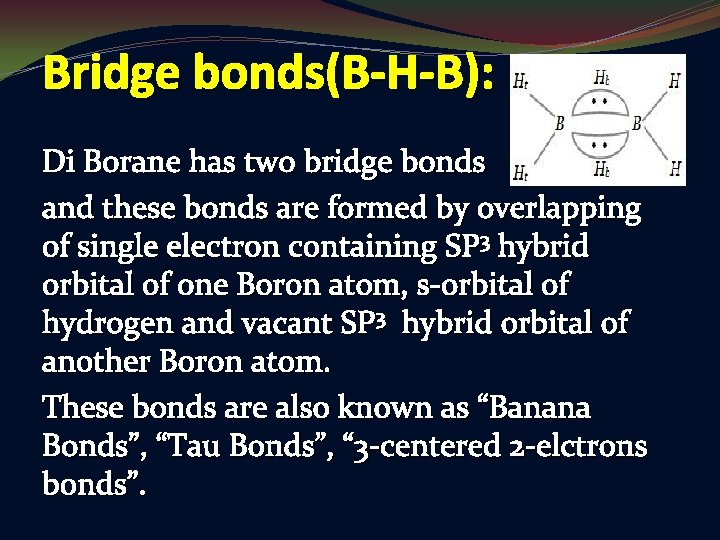
Bridge bonds(B-H-B): Di Borane has two bridge bonds and these bonds are formed by overlapping of single electron containing SP 3 hybrid orbital of one Boron atom, s-orbital of hydrogen and vacant SP 3 hybrid orbital of another Boron atom. These bonds are also known as “Banana Bonds”, “Tau Bonds”, “ 3 -centered 2 -elctrons bonds”.

Terminal bonds(B-H): Di Borane has Four Terminal Bonds and these bonds are formed by overlapping of single electron containing SP 3 hybrid orbital of Boron and S orbital of Hydrogen.

Higher Boranes �The number of Boron atoms more in Boranes are called higher boranes. They are obtained by heating diborane with hydrogen at different temperatures and pressure. �The structures of higher boranes are confirmed by X-ray diffraction.

The reactivity of higher boranes decreases with increase in number of boron atoms. Generally in higher boranes, there are four different types of bonds: i. B-H-B bonds, ii. B-B-B bonds, iii. B-B bonds, iv. B-H bonds
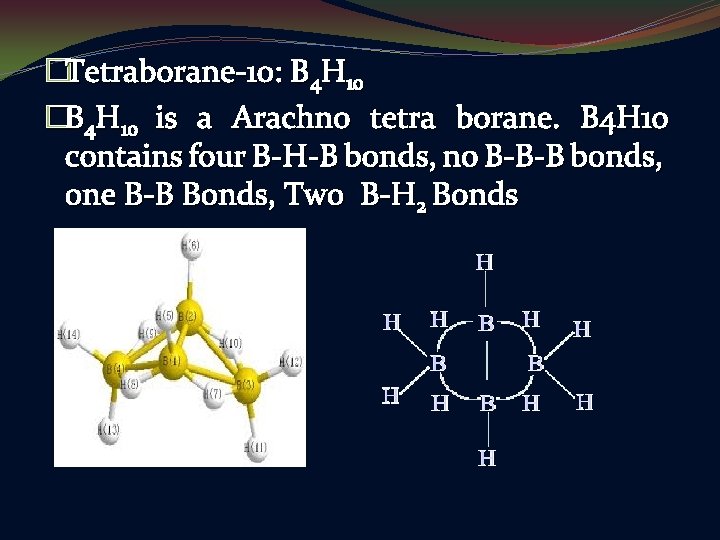
�Tetraborane-10: B 4 H 10 �B 4 H 10 is a Arachno tetra borane. B 4 H 10 contains four B-H-B bonds, no B-B-B bonds, one B-B Bonds, Two B-H 2 Bonds
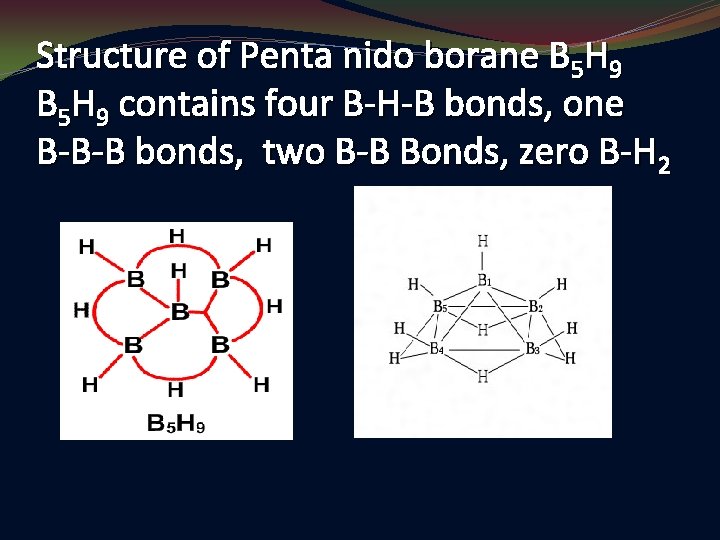
Structure of Penta nido borane B 5 H 9 contains four B-H-B bonds, one B-B-B bonds, two B-B Bonds, zero B-H 2
- Slides: 13