Structure of an Atom What makes up an
- Slides: 9

Structure of an Atom What makes up an atom? What is inside the atom?

What Is an Atom? Atom building blocks of matter. have a nucleus surrounded by an electron cloud Atoms are composed of smaller subatomic particles in an atom. A. ) proton B. ) neutron C. ) electron Each element is composed of one type of atom and can not be broken down into a simpler substance

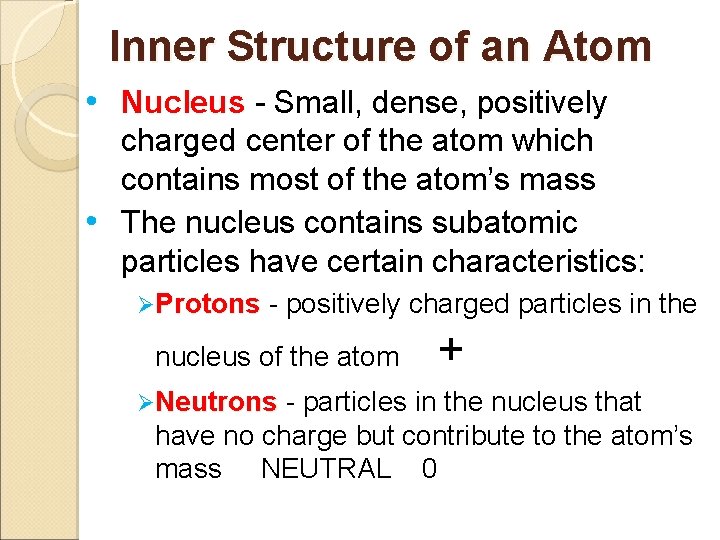
Inner Structure of an Atom • Nucleus - Small, dense, positively charged center of the atom which contains most of the atom’s mass • The nucleus contains subatomic particles have certain characteristics: ØProtons - positively charged particles in the nucleus of the atom ØNeutrons - + particles in the nucleus that have no charge but contribute to the atom’s mass NEUTRAL 0

Outer Structure of an Atom ●Electron cloud - an area encircling the nucleus where electrons are likely to be found ●The cloud contains energy levels Ø electrons are negatively charged particles located in specific energy levels

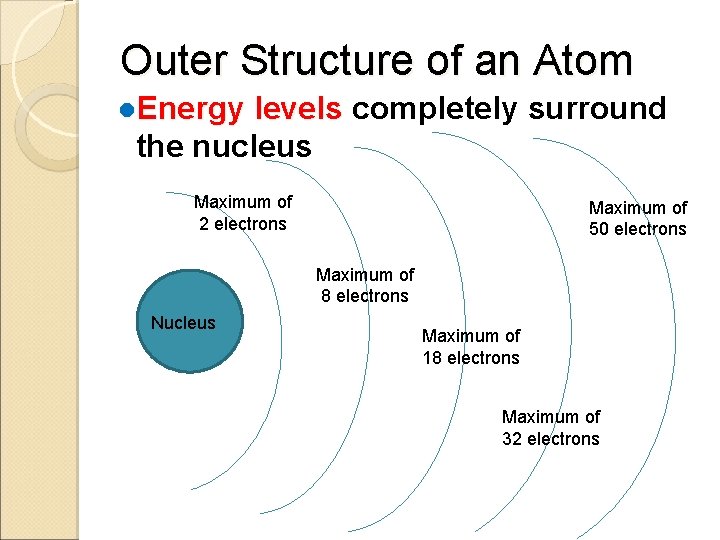
Outer Structure of an Atom ●Energy levels completely surround the nucleus Maximum of 2 electrons Maximum of 50 electrons Maximum of 8 electrons Nucleus Maximum of 18 electrons Maximum of 32 electrons

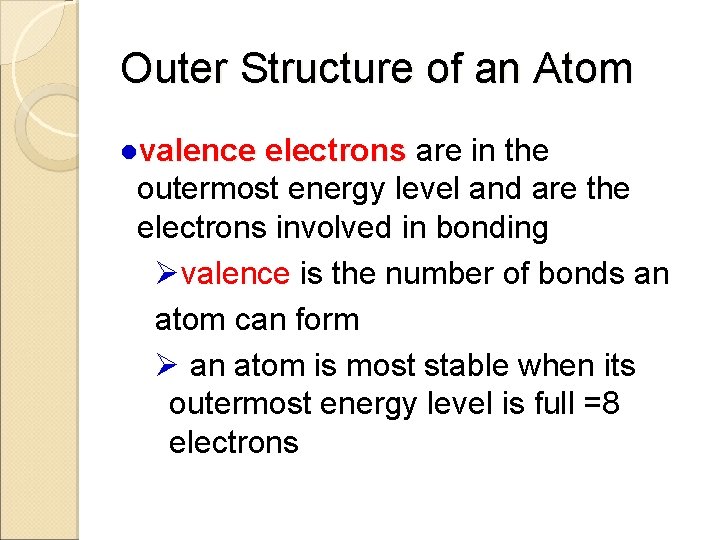
Outer Structure of an Atom ●valence electrons are in the outermost energy level and are the electrons involved in bonding Øvalence is the number of bonds an atom can form Ø an atom is most stable when its outermost energy level is full =8 electrons

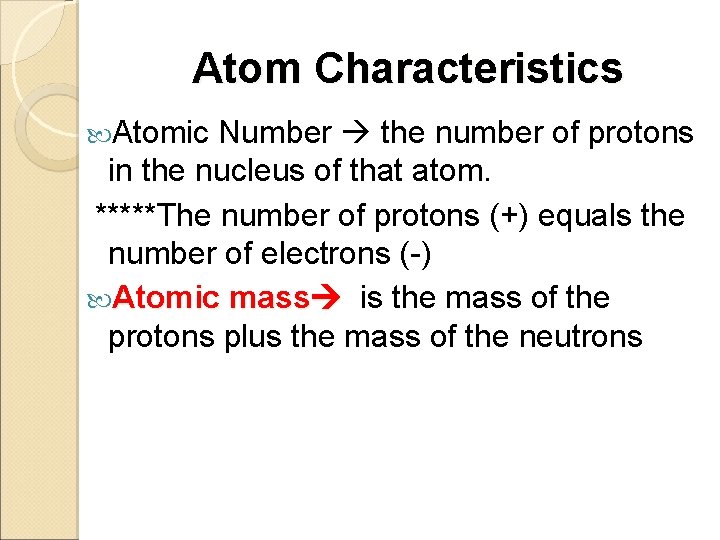
Atom Characteristics Atomic Number the number of protons in the nucleus of that atom. *****The number of protons (+) equals the number of electrons (-) Atomic mass is the mass of the protons plus the mass of the neutrons

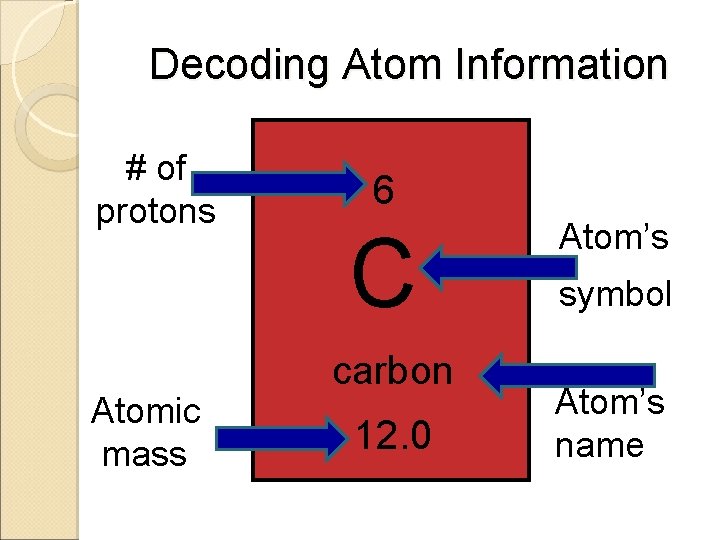
Decoding Atom Information # of protons Atomic mass 6 C carbon 12. 0 Atom’s symbol Atom’s name

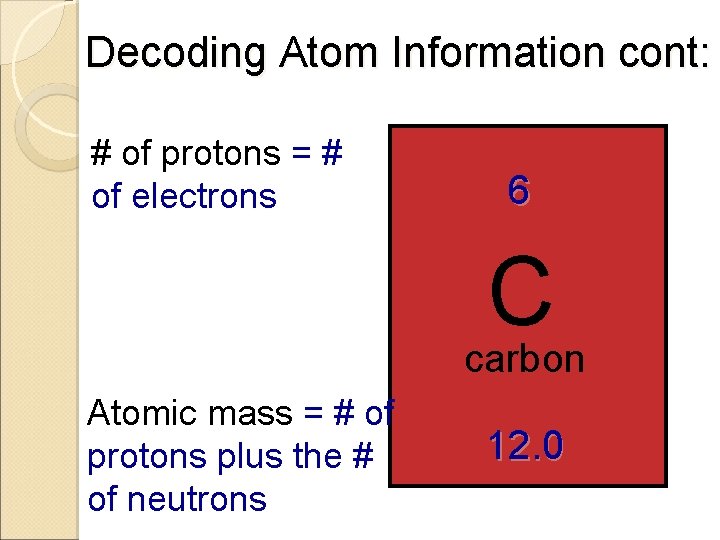
Decoding Atom Information cont: # of protons = # of electrons 6 C carbon Atomic mass = # of protons plus the # of neutrons 12. 0