Structure of an Atom What Is an Atom








- Slides: 18

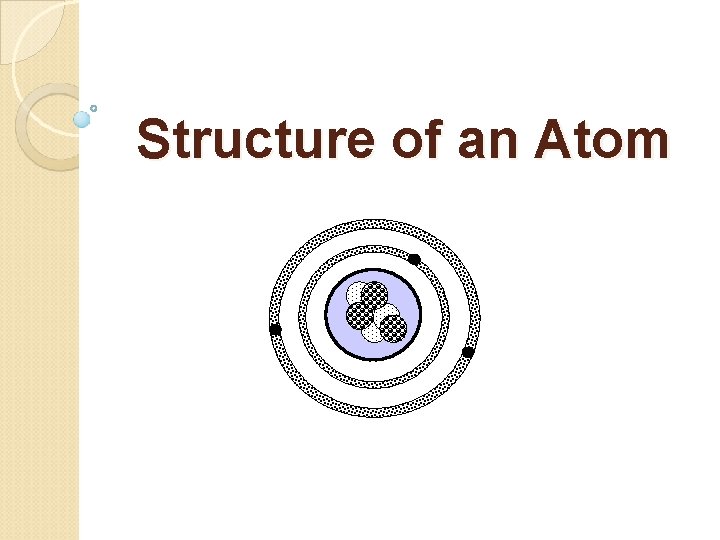
Structure of an Atom

What Is an Atom? Atoms are often referred to as the building blocks of matter. Each element on the periodic table is composed of one type of atom and cannot be broken down into a simpler substance.

What Is an Atom? Atoms are composed of smaller subatomic particles such as the proton, neutron, and electron. Atoms contain a nucleus surrounded by an electron cloud that consists of one or more energy levels.

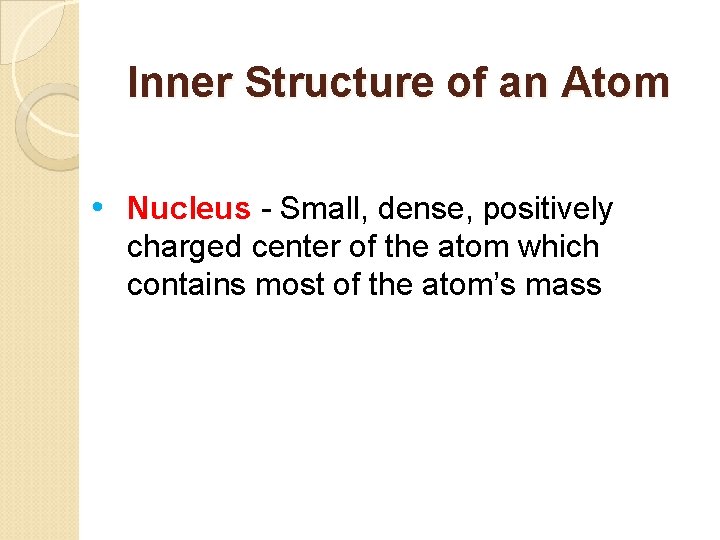
Inner Structure of an Atom • Nucleus - Small, dense, positively charged center of the atom which contains most of the atom’s mass

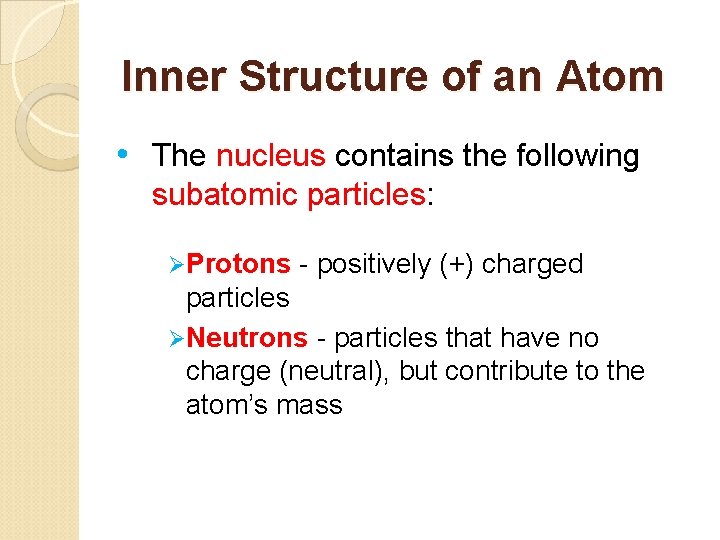
Inner Structure of an Atom • The nucleus contains the following subatomic particles: ØProtons - positively (+) charged particles ØNeutrons - particles that have no charge (neutral), but contribute to the atom’s mass

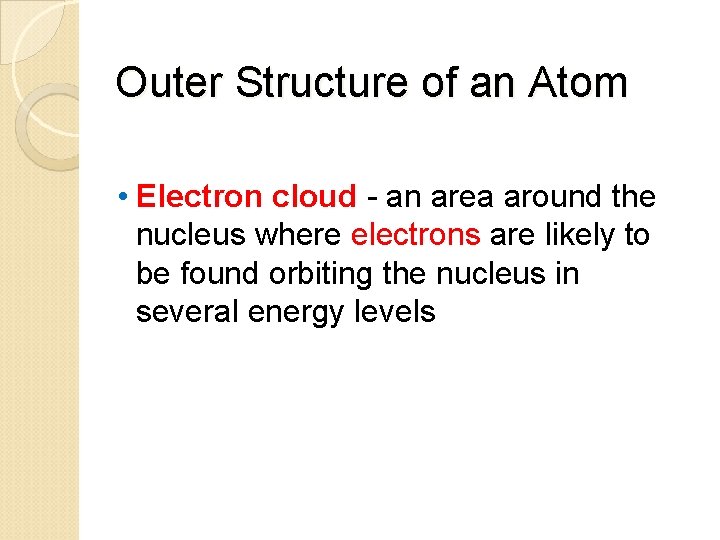
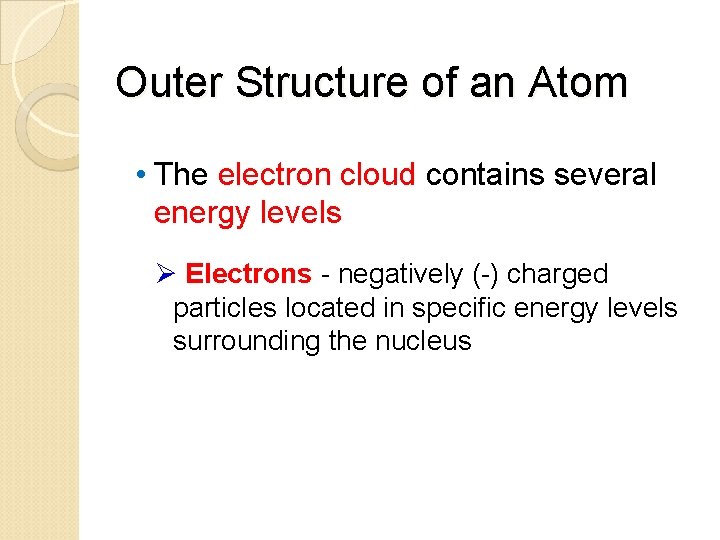
Outer Structure of an Atom • Electron cloud - an area around the nucleus where electrons are likely to be found orbiting the nucleus in several energy levels

Outer Structure of an Atom • The electron cloud contains several energy levels Ø Electrons - negatively (-) charged particles located in specific energy levels surrounding the nucleus

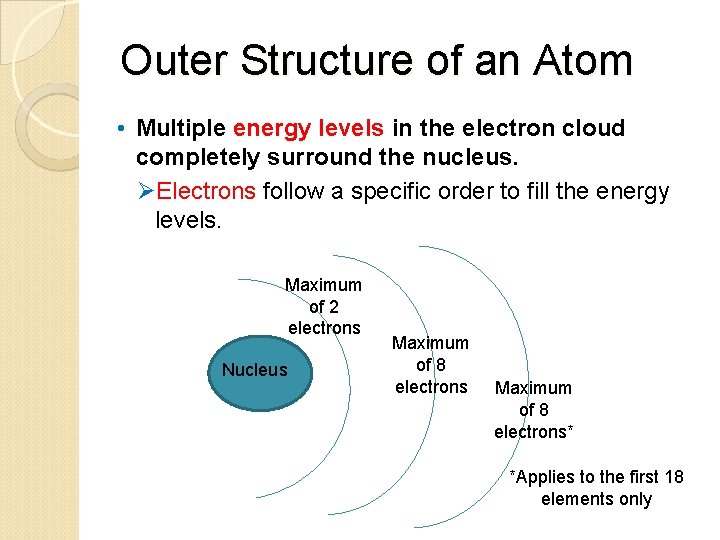
Outer Structure of an Atom • Multiple energy levels in the electron cloud completely surround the nucleus. ØElectrons follow a specific order to fill the energy levels. Maximum of 2 electrons Nucleus Maximum of 8 electrons* *Applies to the first 18 elements only

Outer Structure of an Atom • The electrons in the outermost energy level are called valence electrons • We will go into more detail about the importance of valence electrons in our next unit.

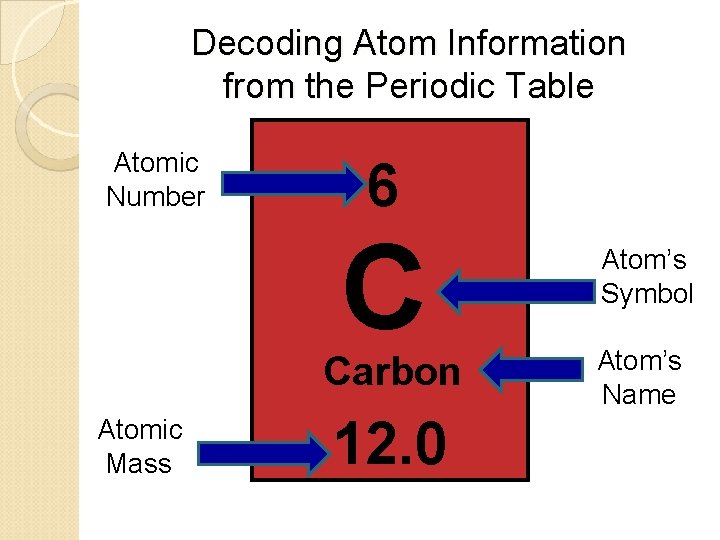
Decoding Atom Information from the Periodic Table Atomic Number 6 C Carbon Atomic Mass 12. 0 Atom’s Symbol Atom’s Name

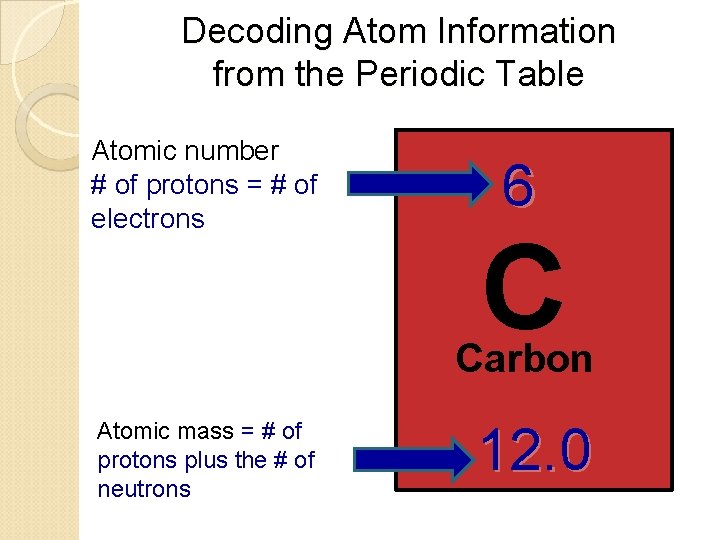
Decoding Atom Information from the Periodic Table Atomic number # of protons = # of electrons 6 C Carbon Atomic mass = # of protons plus the # of neutrons 12. 0

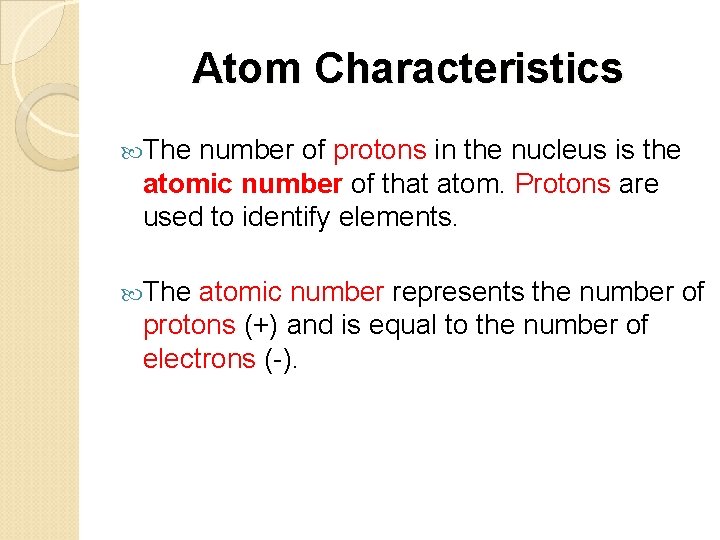
Atom Characteristics The number of protons in the nucleus is the atomic number of that atom. Protons are used to identify elements. The atomic number represents the number of protons (+) and is equal to the number of electrons (-).

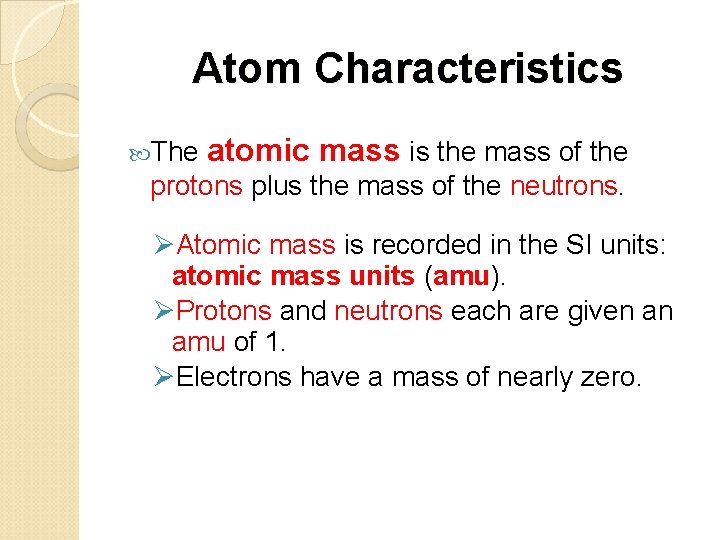
Atom Characteristics The atomic mass is the mass of the protons plus the mass of the neutrons. ØAtomic mass is recorded in the SI units: atomic mass units (amu). ØProtons and neutrons each are given an amu of 1. ØElectrons have a mass of nearly zero.

Con’t Notes The proton within an atom contains a positive electrical charge. A nucleus is the central core of an atom. All atoms are electrically neutral. Atoms become electrically charged when they lose and gain electrons.

The nuclei of atoms are electrically positive. The central core of an atom consists of neutrons and protons. The smallest particle of any element that still has the properties of that element is called an atom. A proton and neutron are similar in mass. A neutron does not have either a positive or negative charge.

The nucleus is made up of protons and neutrons. Positive and neutral particles are in the center and surrounded by an electron cloud. Valence electrons are located in the occupied outermost energy level. The mass number is equal to the sum of the protons plus the neutrons of an atom on the periodic table. All atoms of a certain element have the same number of protons and electrons.

The electron within an atom contains a negative electric charge. The nuclei of atoms have a positive charge because protons are present. 2 electrons are in the energy level closest to the nucleus.

The negatively charged particle that spins outside the nucleus of the atom is called the electron. The number of protons of an atom can be determined by looking at the atomic number. Mass number is the sum of protons and neutrons in an atom.