Structure and function of hemoglobin Hemoglobin Hb n

Structure and function of hemoglobin

Hemoglobin (Hb) n n A hemeprotein found only in red blood cells Oxygen transport function Contains heme as prosthetic group Heme reversibly binds to oxygen

The heme group n n A complex of protoporphyrin IX and ferrous iron (Fe 2+) Fe 2+ is present in the center of the heme Fe 2+ binds to four nitrogen atoms of the porphyrin ring Forms two additional bonds with: Histidine residue of globin chain n Oxygen n
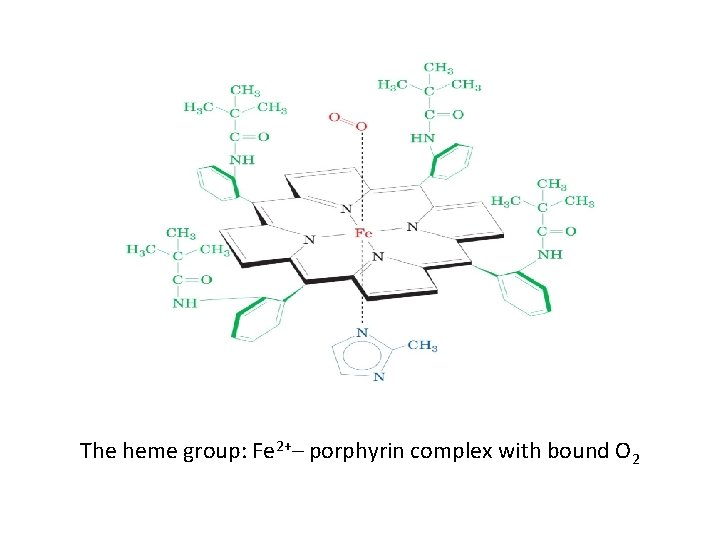
The heme group: Fe 2+– porphyrin complex with bound O 2
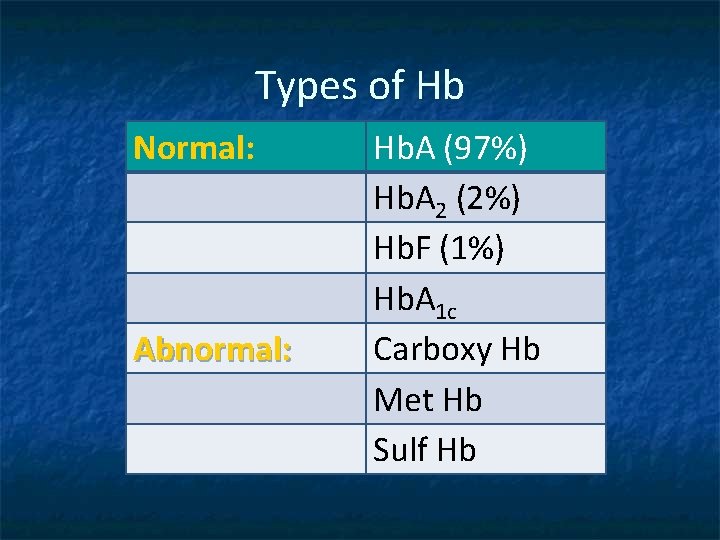
Types of Hb Normal: Abnormal: Hb. A (97%) Hb. A 2 (2%) Hb. F (1%) Hb. A 1 c Carboxy Hb Met Hb Sulf Hb
Hemoglobin A (Hb. A) n n Major Hb in adults Composed of four polypetide chains: n n n Two α and two β chains Contains two dimers of ab subunits Held together by non-covalent interactions Each chain is a subunit with a heme group in the center that carries oxygen A Hb molecule contains 4 heme groups and carries 4 moelcules of O 2
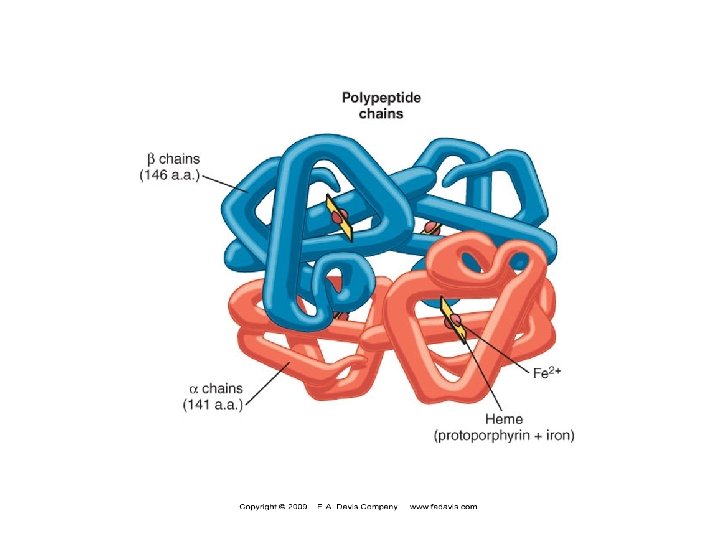
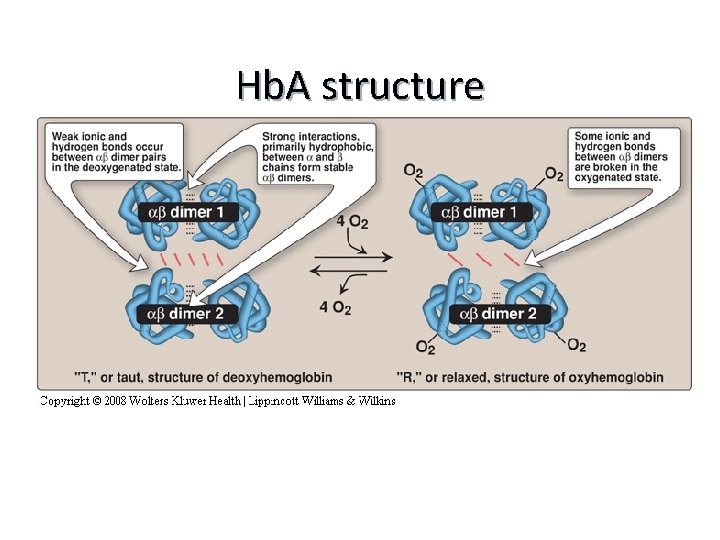
Hb. A structure
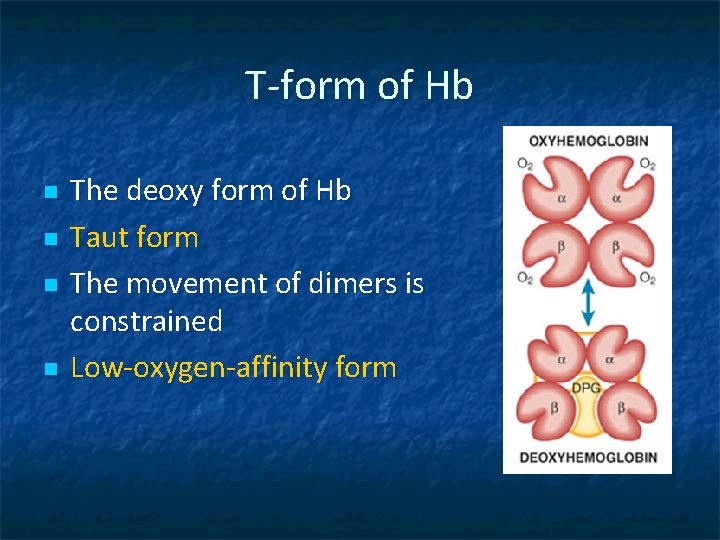
T-form of Hb n n The deoxy form of Hb Taut form The movement of dimers is constrained Low-oxygen-affinity form
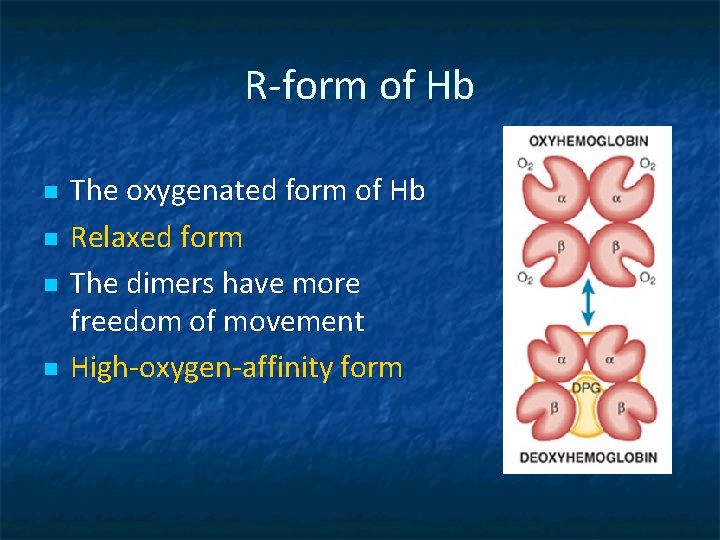
R-form of Hb n n The oxygenated form of Hb Relaxed form The dimers have more freedom of movement High-oxygen-affinity form

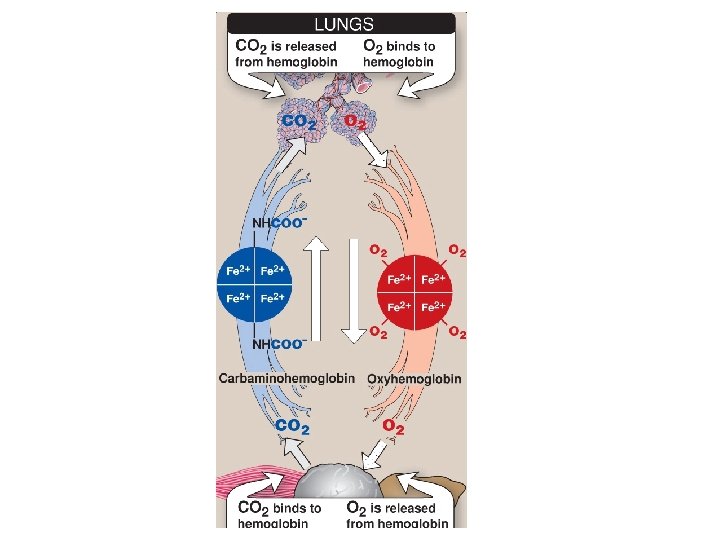
Hemoglobin function n Carries oxygen from the lungs to tissues Carries carbon dioxide from tissues back to the lungs Normal level (g/d. L): • • Males: 14 -16 Females: 13 -15

Factors affecting oxygen binding n Three allosteric effectors: n n p. O 2 (partial oxygen pressure) p. H of the environment p. CO 2 (partial carbon dioxide pressure) Availability of 2, 3 -bisphoglycerate
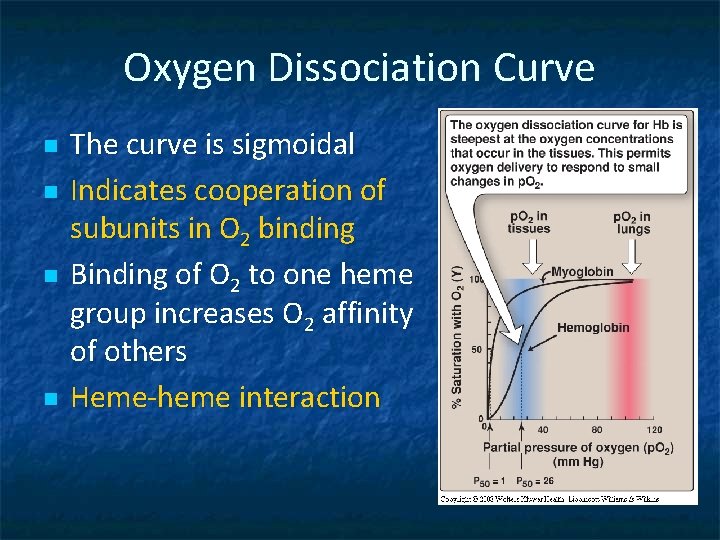
Oxygen Dissociation Curve n n The curve is sigmoidal Indicates cooperation of subunits in O 2 binding Binding of O 2 to one heme group increases O 2 affinity of others Heme-heme interaction

P 50 n Indicates affinity of Hb to O 2 n P 50(mm Hg): the pressure at which Hb is 50% saturated with O 2 n High affinity slow unloading of O 2 n Low affinity fast unloading of O 2 n Lung p. O 2 is 100 mm Hb saturation 100% n Tissue p. O 2 is 40 mm Hb saturation reduces n Hence O 2 is delivered to tissues
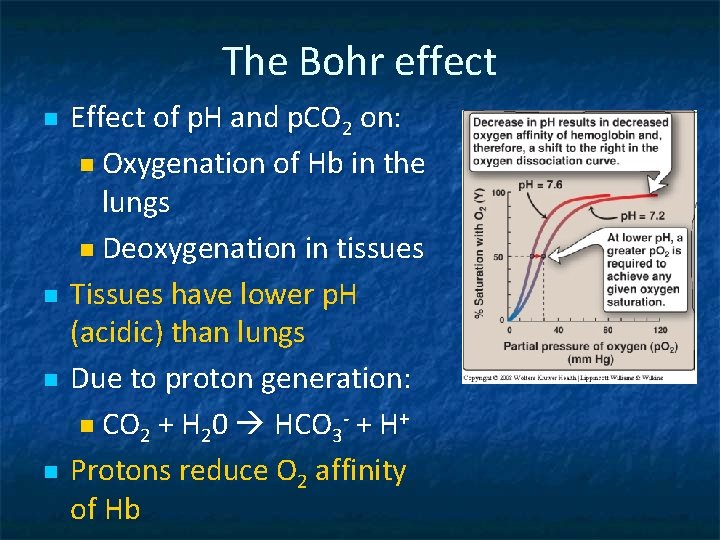
The Bohr effect n n Effect of p. H and p. CO 2 on: n Oxygenation of Hb in the lungs n Deoxygenation in tissues Tissues have lower p. H (acidic) than lungs Due to proton generation: n CO 2 + H 20 HCO 3 - + H+ Protons reduce O 2 affinity of Hb
The Bohr Effect n n n Causing easier O 2 release into the tissues The free Hb binds to two protons Protons are released and react with HCO 3 – to form CO 2 gas (HCO 3 - + H+ CO 2 + H 20 ) The proton-poor Hb now has greater affinity for O 2 (in lungs) The Bohr effect removes insoluble CO 2 from blood stream Produces soluble bicarbonate
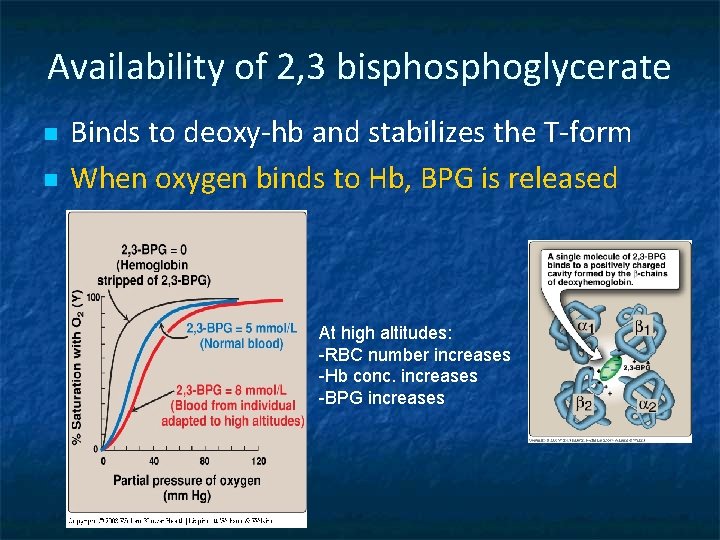
Availability of 2, 3 bisphoglycerate n n Binds to deoxy-hb and stabilizes the T-form When oxygen binds to Hb, BPG is released At high altitudes: -RBC number increases -Hb conc. increases -BPG increases

High altitude and O 2 affinity n In hypoxia and high altitude 2, 3 BPG levels rise n This decreases O 2 affinity of Hb n Thus increases O 2 delivery to tissues n

High O 2 affinity is due to: n Alkalosis n High levels of Hb F n Multiple transfusion of 2, 3 DPG-depleted blood
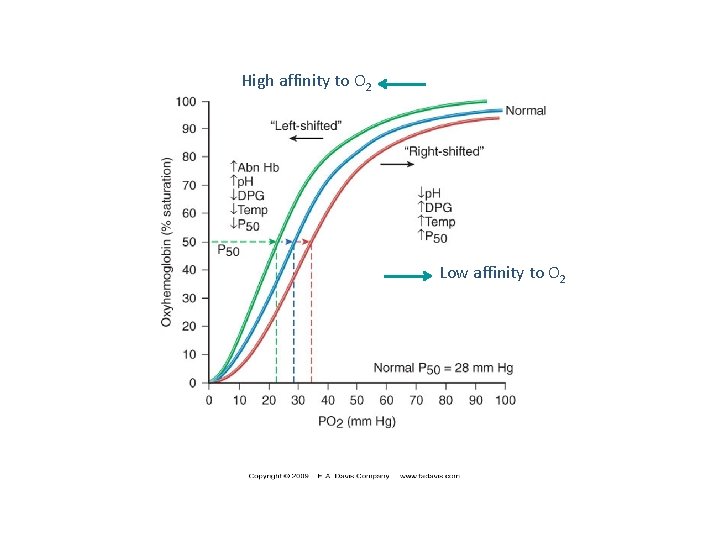
High affinity to O 2 Low affinity to O 2

Fetal Hemoglobin (Hb. F) n n Major hemoglobin found in the fetus and newborn Tetramer with two a and two g chains Higher affinity for O 2 than HBA Transfers O 2 from maternal to fetal circulation across placenta

Hb. A 2 n n n Appears ~12 weeks after birth Constitutes ~2% of total Hb Composed of two a and two d globin chains
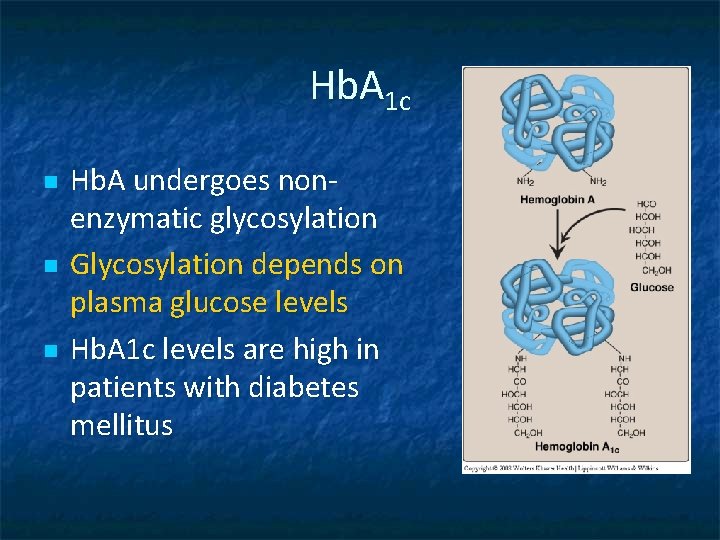
Hb. A 1 c n n n Hb. A undergoes nonenzymatic glycosylation Glycosylation depends on plasma glucose levels Hb. A 1 c levels are high in patients with diabetes mellitus

Abnormal Hbs Unable to transport O 2 due to abnormal structure n Carboxy-Hb: CO replaces O 2 and binds 200 X tighter than O 2 (in smokers) n Met-Hb: Contains oxidized Fe 3+ (~2%) that cannot carry O 2 n Sulf-HB: Forms due to high sulfur levels in blood (irreversible reaction)
- Slides: 25