Structure and function of cell components carbohydrates fats
Structure and function of cell components • carbohydrates • fats (lipids) • proteins • nucleic acids • membranes • cytoskeleton

Introduction • elements/chemicals/complexity • carbon essential • functional groups • monomers/polymers • ionised forms (loss of hydrogen) • Macromolecules • Condensation reaction (dehydration synthesis - removing elements of water) • Hydrolysis (addition of elements from water) • Bonding (ionic, covalent, hydrogen, van der vaals)

Introduction • Energy – bonds broken releases energy bonds formed requires energy • Biosynthesis - building molecules (anabolic) • Biodegradation - breaking down molecules (catabolic) • Reversibility - Energy 'neutral' reactions • 3 D shape of molecules • Water - the 'universal' solvent
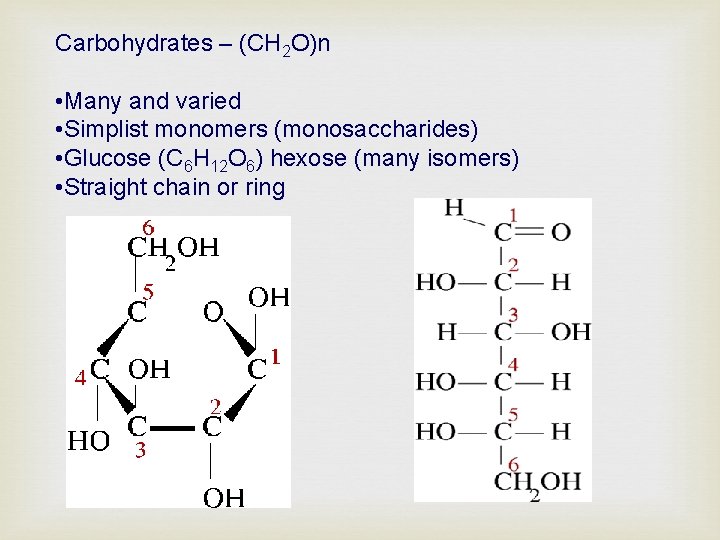
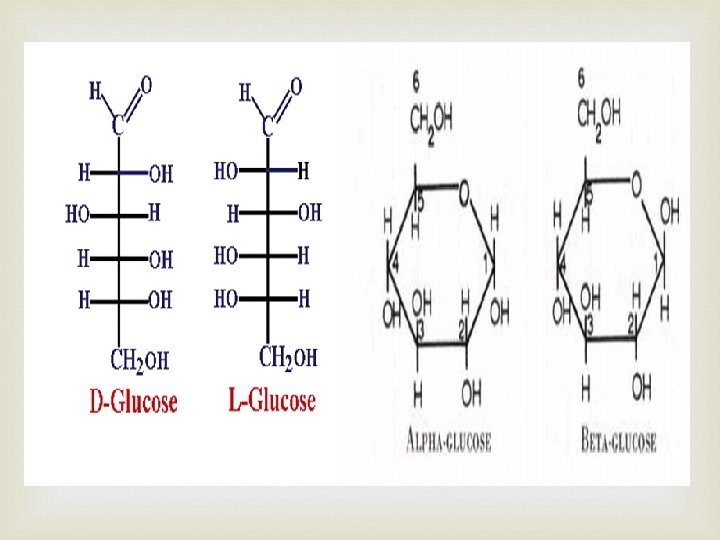
Carbohydrates – (CH 2 O)n • Many and varied • Simplist monomers (monosaccharides) • Glucose (C 6 H 12 O 6) hexose (many isomers) • Straight chain or ring
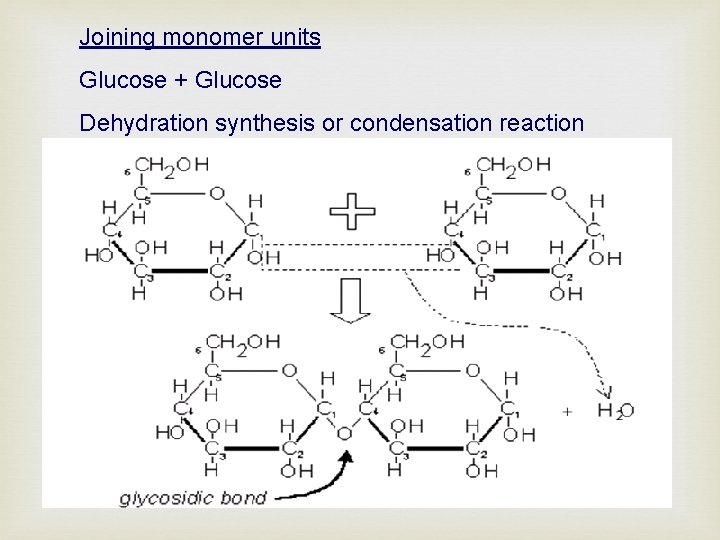
Joining monomer units Glucose + Glucose Dehydration synthesis or condensation reaction

Hexose sugars (monomers) • glucose • fructose • galactose Disaccharides • sucrose = glucose + fructose • lactose = glucose + galactose • maltose = glucose + glucose
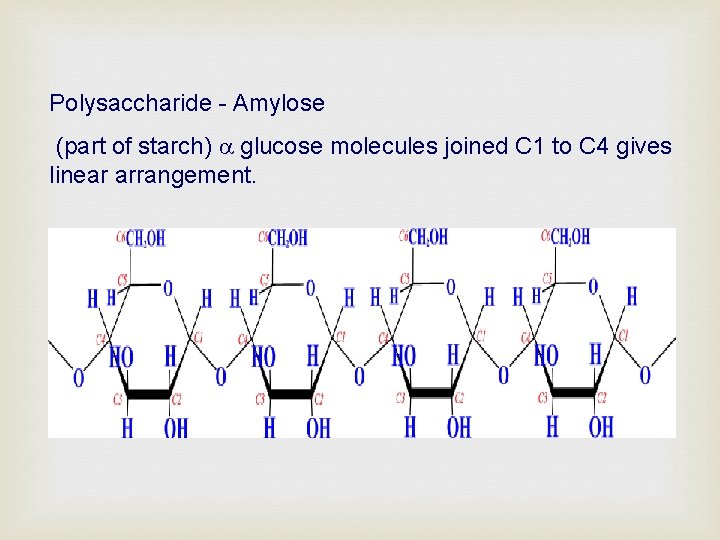
Polysaccharide - Amylose (part of starch) glucose molecules joined C 1 to C 4 gives linear arrangement.
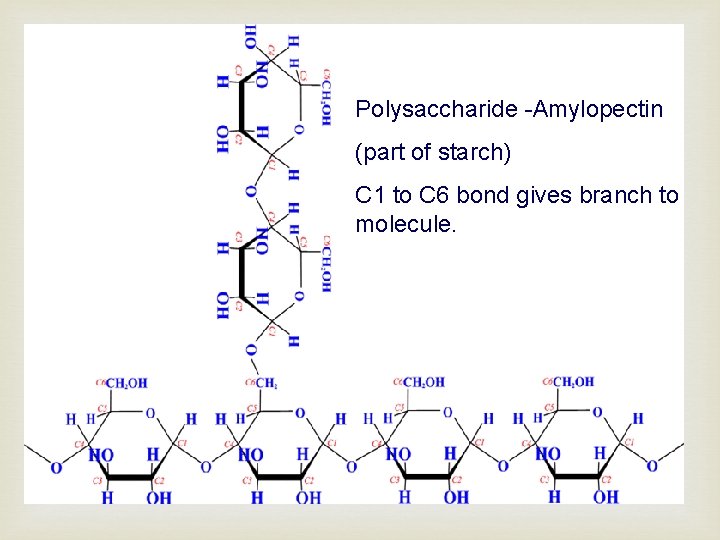
Polysaccharide -Amylopectin (part of starch) C 1 to C 6 bond gives branch to molecule.
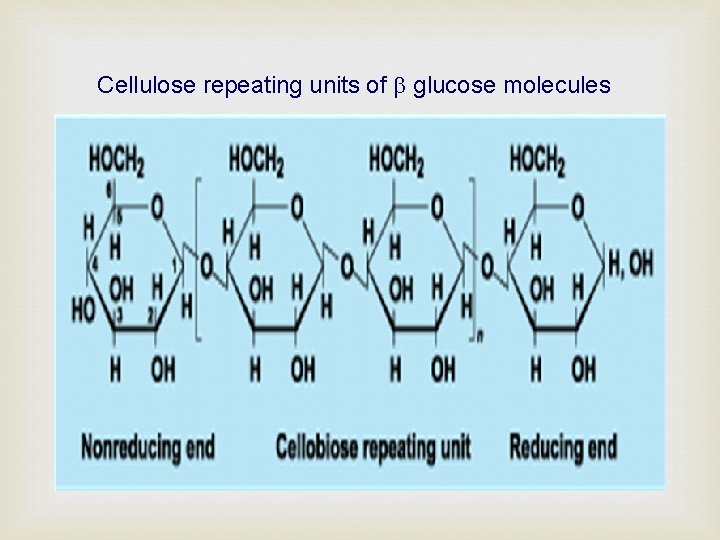
Cellulose repeating units of glucose molecules
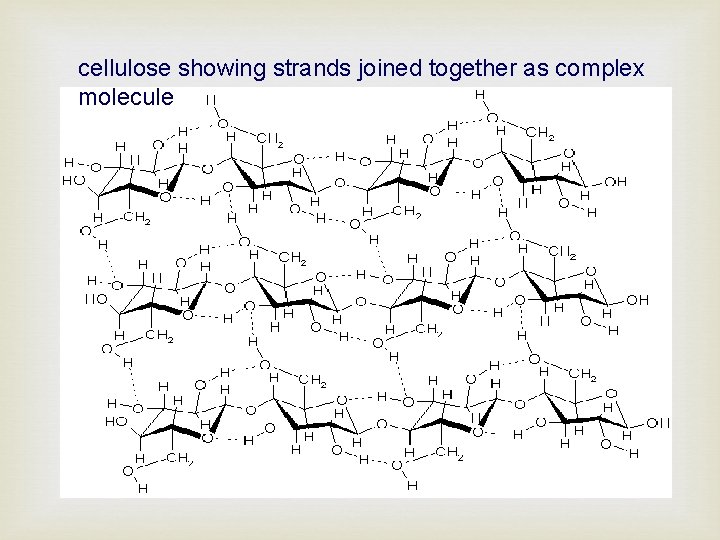
cellulose showing strands joined together as complex molecule
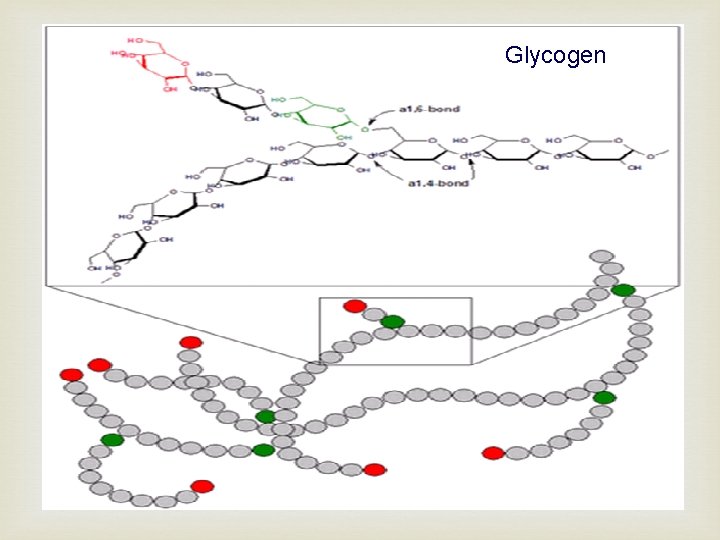
Glycogen

Significance of carbohydrates in organisms • energy budget • energy storage • cell structures • storage and osmoregulation
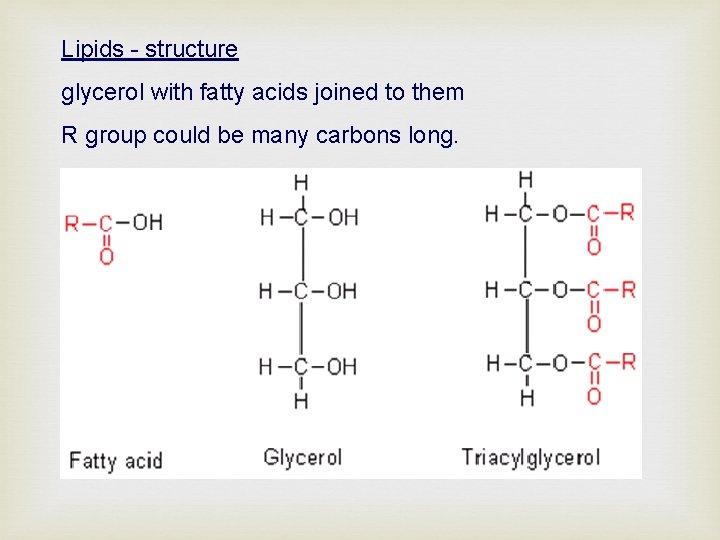
Lipids - structure glycerol with fatty acids joined to them R group could be many carbons long.
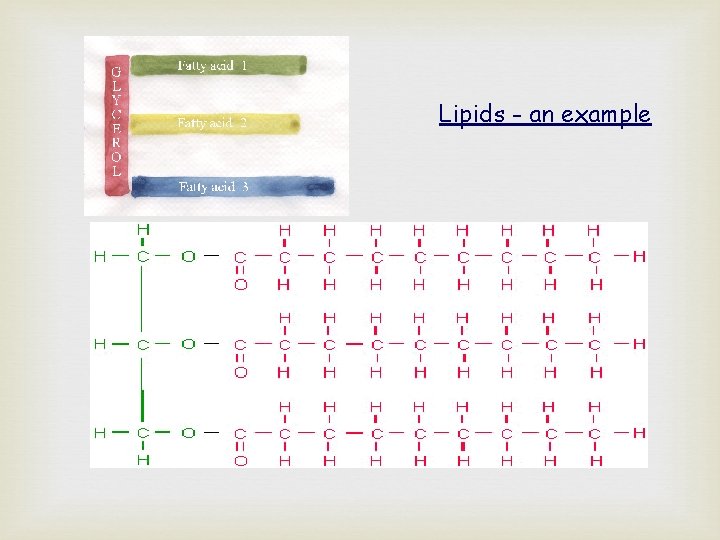
Lipids - an example
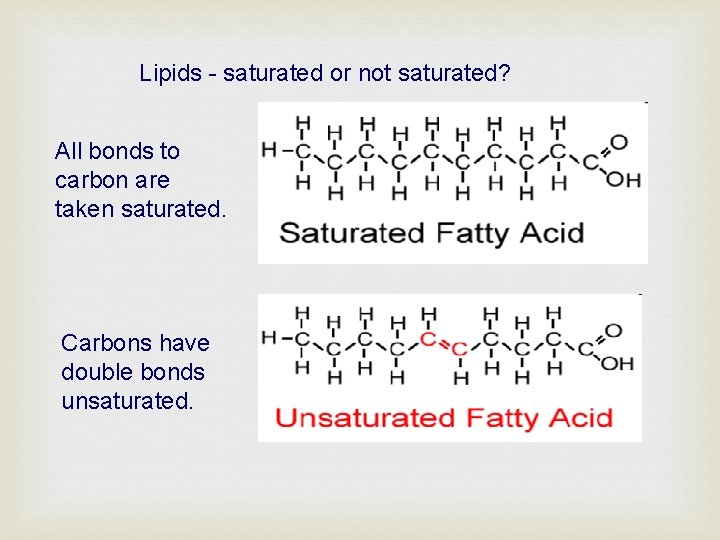
Lipids - saturated or not saturated? All bonds to carbon are taken saturated. Carbons have double bonds unsaturated.
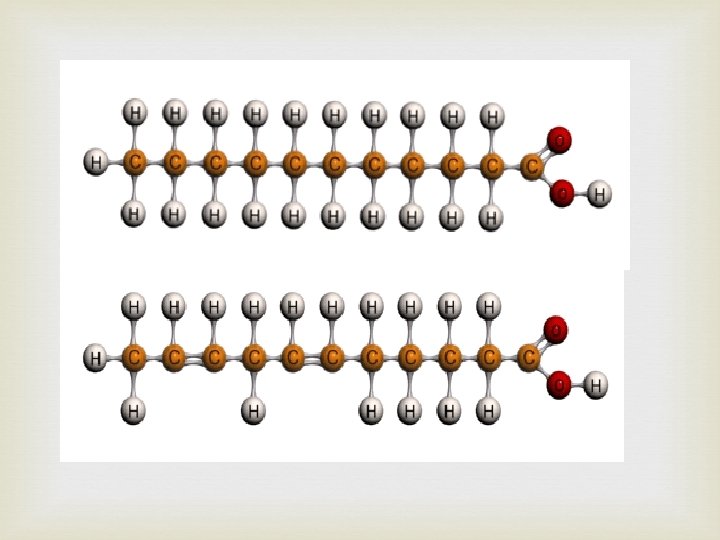
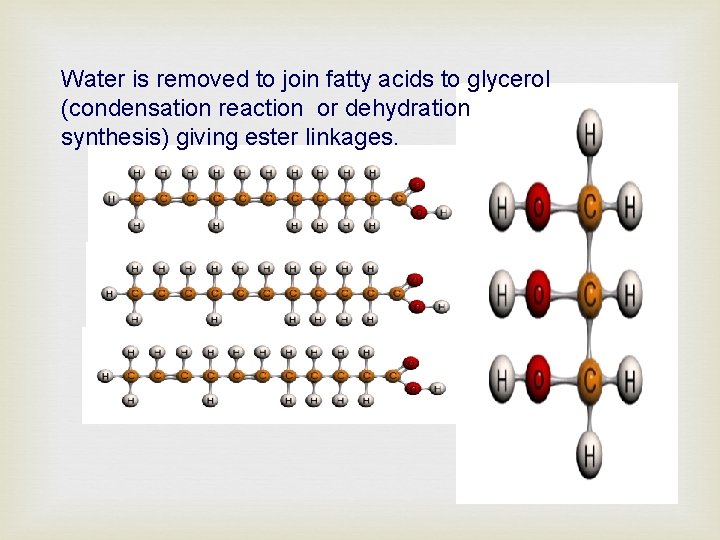
Water is removed to join fatty acids to glycerol (condensation reaction or dehydration synthesis) giving ester linkages.
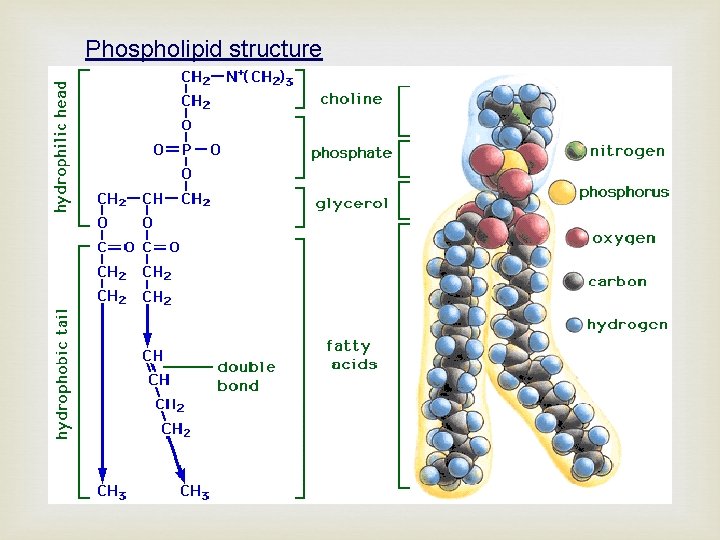
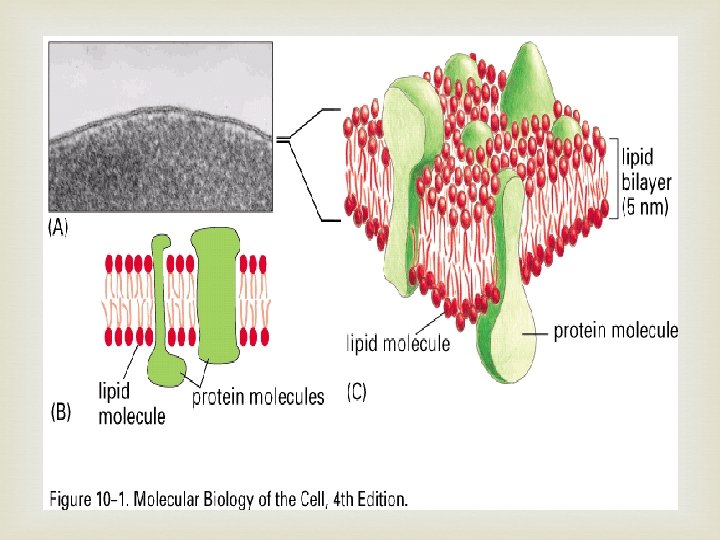
Phospholipid structure
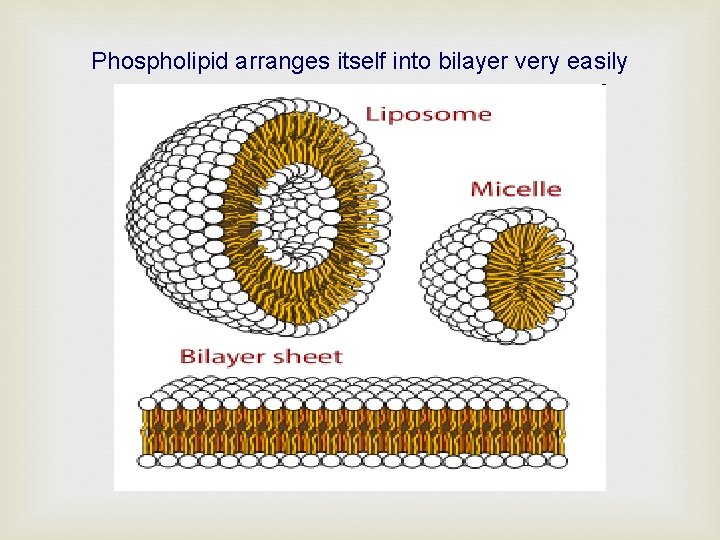
Phospholipid arranges itself into bilayer very easily
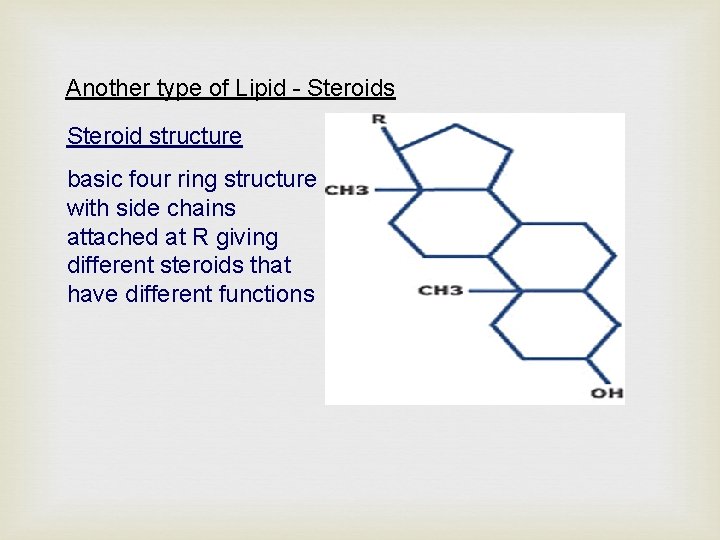
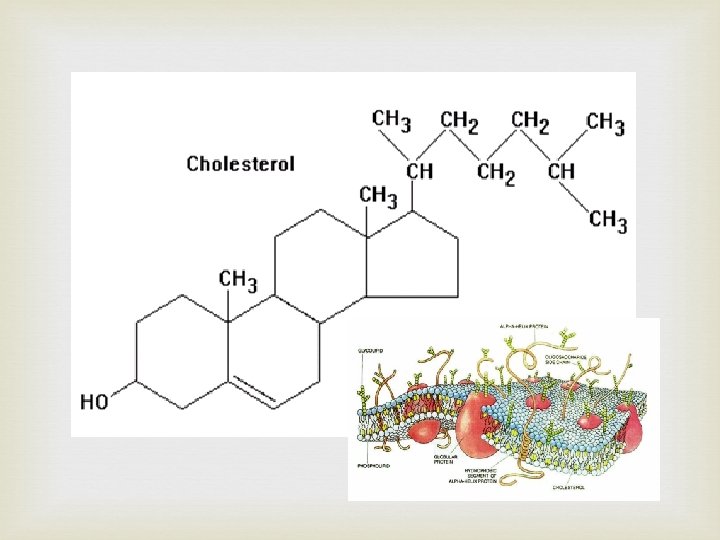
Another type of Lipid - Steroids Steroid structure basic four ring structure with side chains attached at R giving different steroids that have different functions
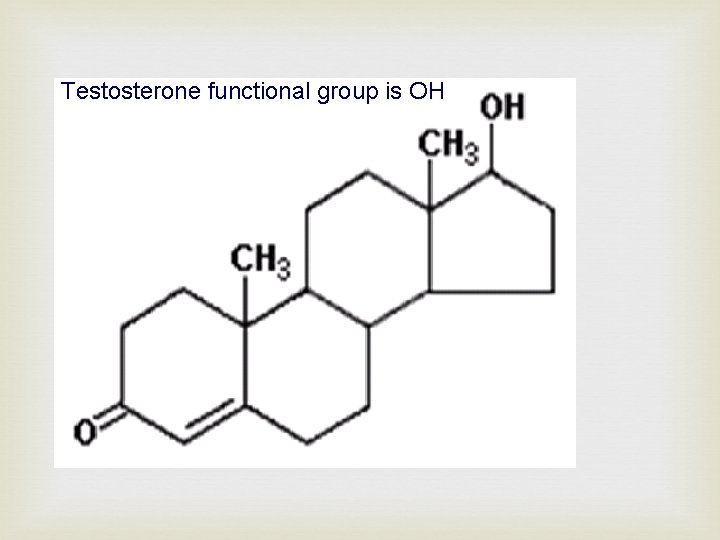
Testosterone functional group is OH
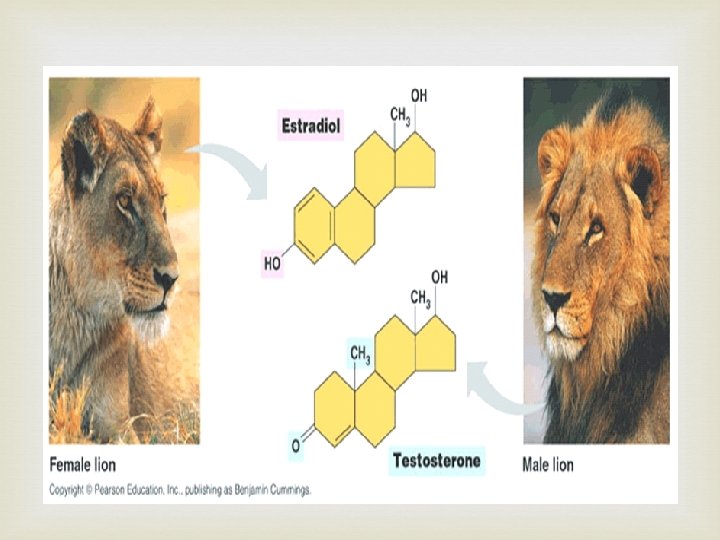

Proteins Basically they are composed of amino acids joined together but there are 4 levels to their structure: • Primary • Secondary • Tertiary • Quaternary
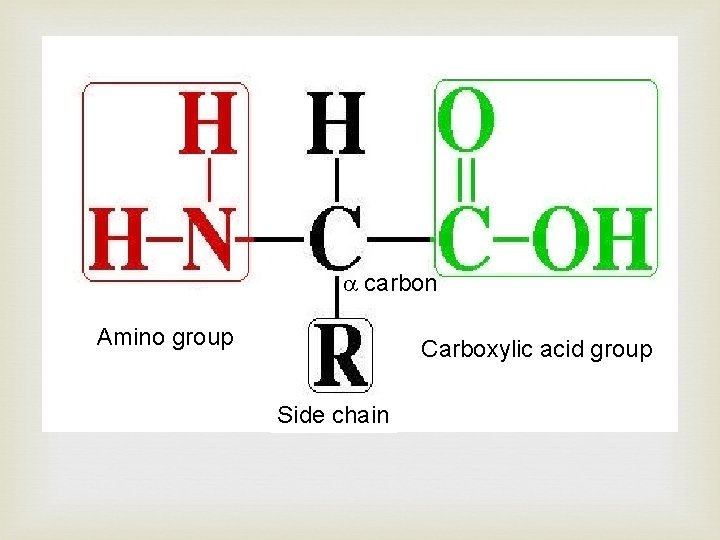
carbon Amino group Carboxylic acid group Side chain

Side chain at R determine the type of amino acid. There are 4 types of side chains giving four main categories of amino acid: • Polar - R group has a distinct charge e. g. OH • Non-polar - R group no overall charge • Acidic - R group has acid (COOH) at end • Basic – R group has amide (NH 2) at end There are combinations of these categories possible such as polar basic and polar acidic
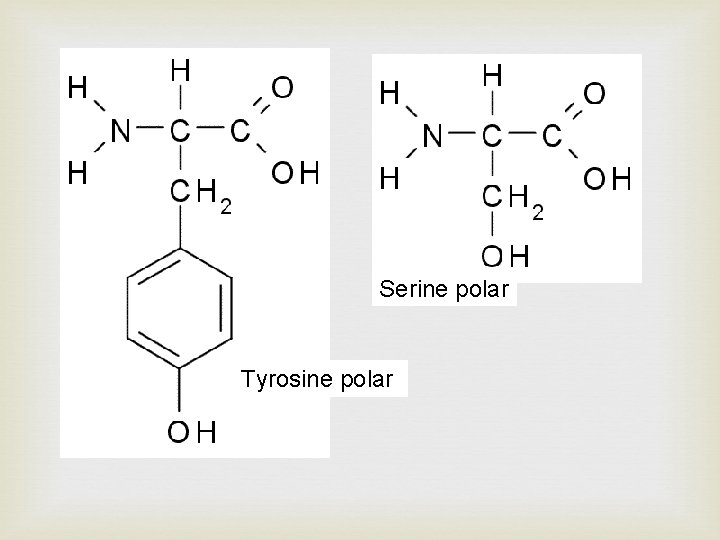
Serine polar Tyrosine polar
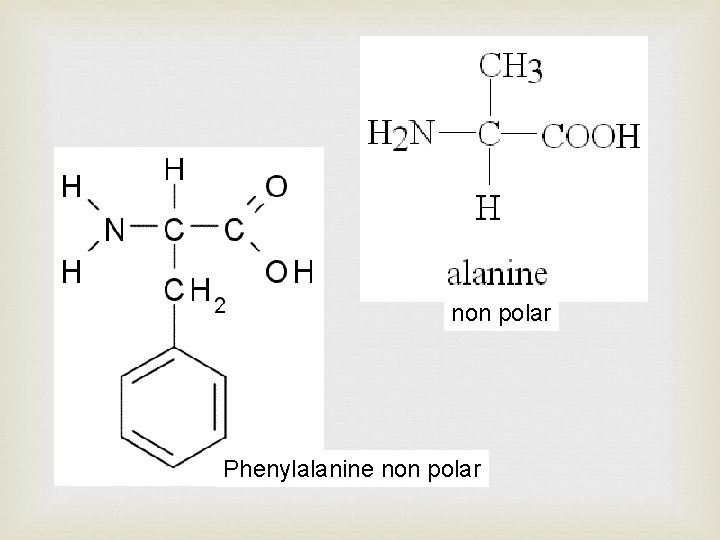
non polar Phenylalanine non polar
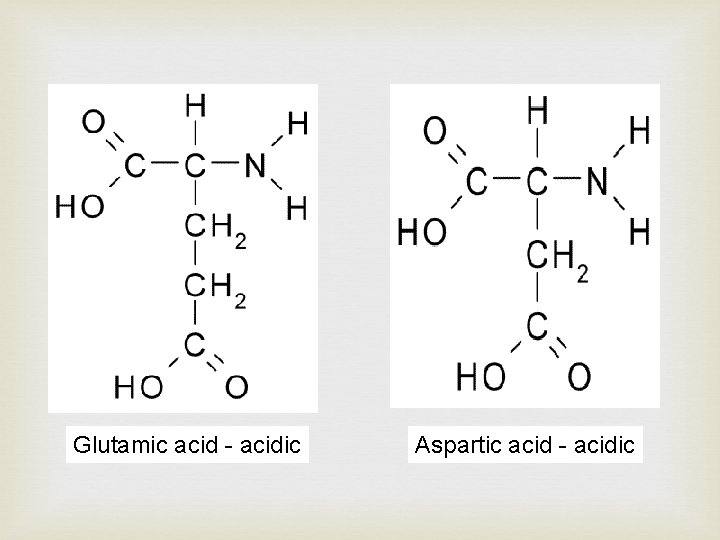
Glutamic acid - acidic Aspartic acid - acidic
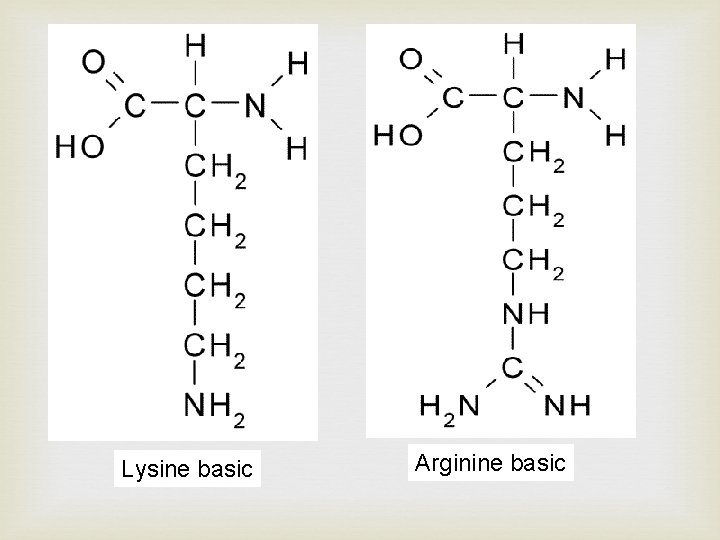
Lysine basic Arginine basic
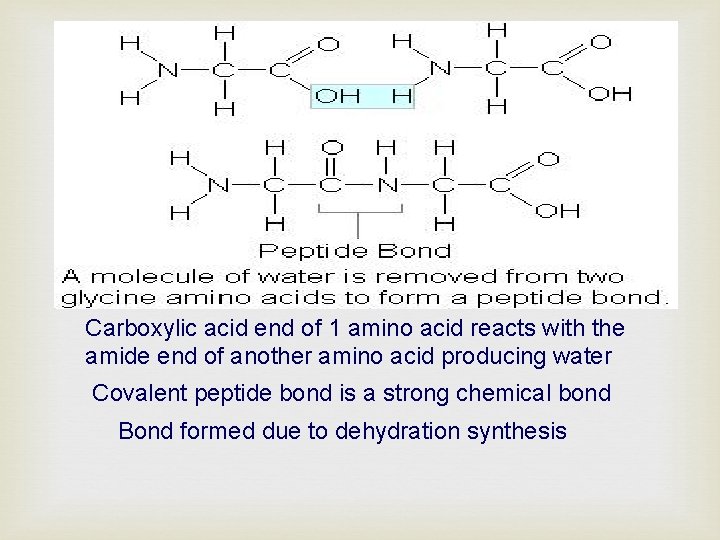
Carboxylic acid end of 1 amino acid reacts with the amide end of another amino acid producing water Covalent peptide bond is a strong chemical bond Bond formed due to dehydration synthesis

Primary Structure Depends on the sequence of amino acids in the polypeptide chain. This is predetermined by the DNA of an organism. Only specific sequences will bring about correct function. Many amino acids joined together by peptide bonds give a polypeptide chain.

Secondary Structure Is related to how the polypeptide chain is folded. It is mainly concerned with hydrogen bonds between C=O and N-H groups. There are 2 common types of folding: -helix -sheet
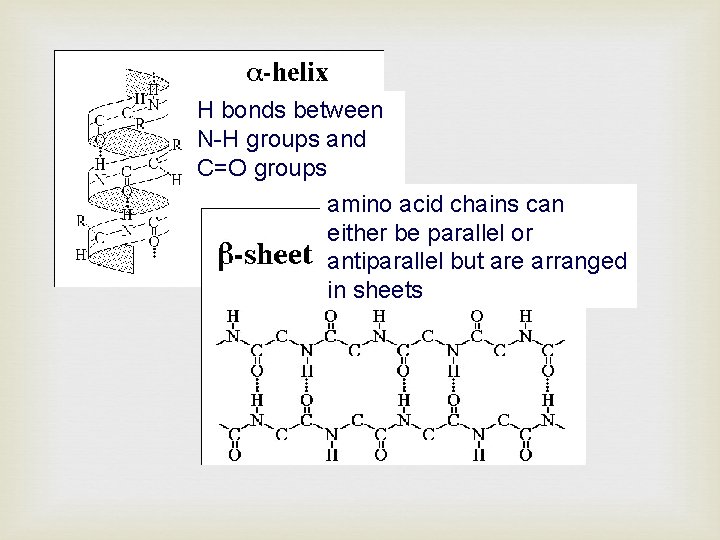
H bonds between N-H groups and C=O groups amino acid chains can either be parallel or antiparallel but are arranged in sheets
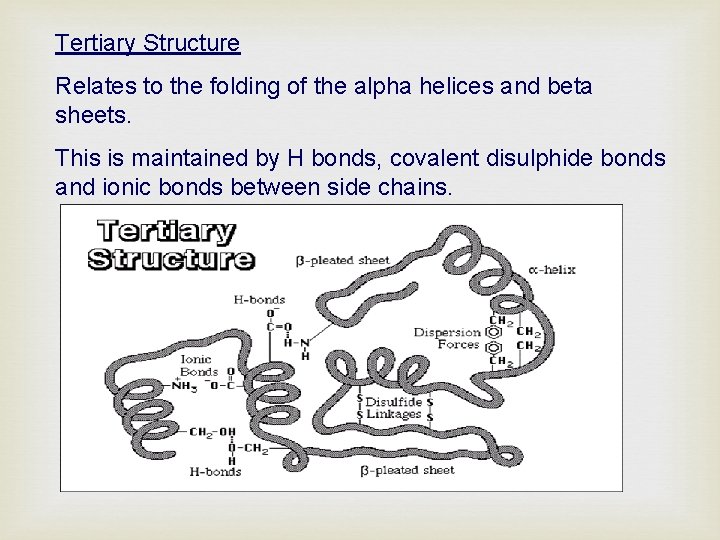
Tertiary Structure Relates to the folding of the alpha helices and beta sheets. This is maintained by H bonds, covalent disulphide bonds and ionic bonds between side chains.
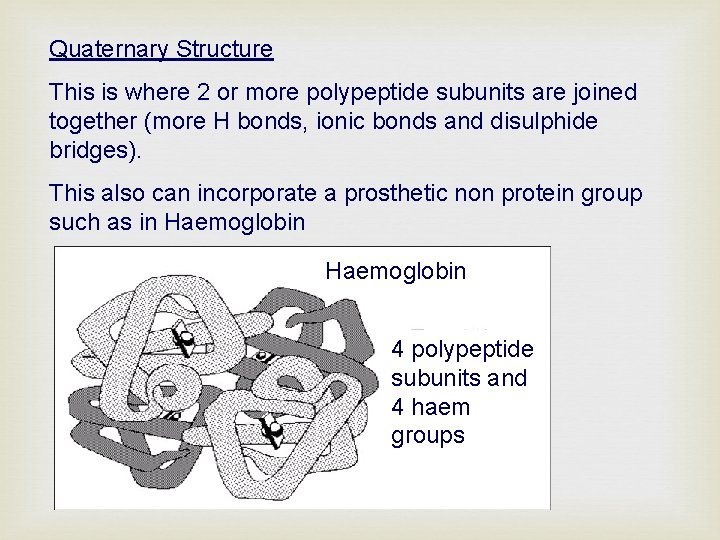
Quaternary Structure This is where 2 or more polypeptide subunits are joined together (more H bonds, ionic bonds and disulphide bridges). This also can incorporate a prosthetic non protein group such as in Haemoglobin 4 polypeptide subunits and 4 haem groups
Functions of Protein • Catalytic (enzymes) - speed up chemical reactions. • Structural - cell membrane, tissues, etc. • Messenger (hormones) - chemical messengers within cells and between cells • Carriers - proteins transporting chemicals into and out of cells.
- Slides: 39