Structure and Bonding Revision Summary Bonding Covalent l
Structure and Bonding Revision Summary

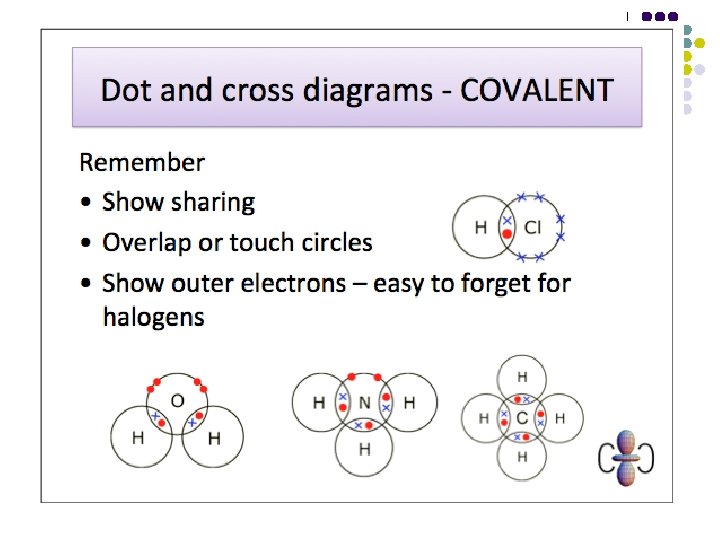
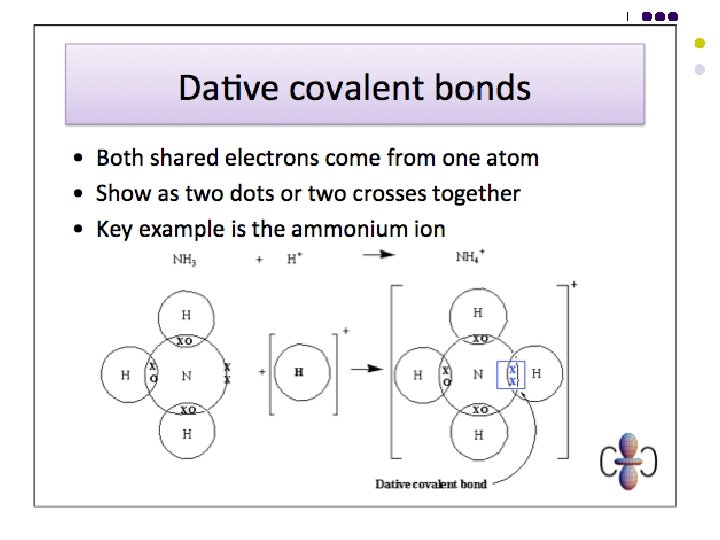
Bonding - Covalent l l A covalent bond is the electrostatic force of attraction of two nuclei towards a shared pair of electrons between them, one electron in the shared pair coming from each atom. A dative covalent bond is the electrostatic force of attraction of two nuclei towards a shared pair of electrons between them, both electrons in the shared pair coming from the same atom.

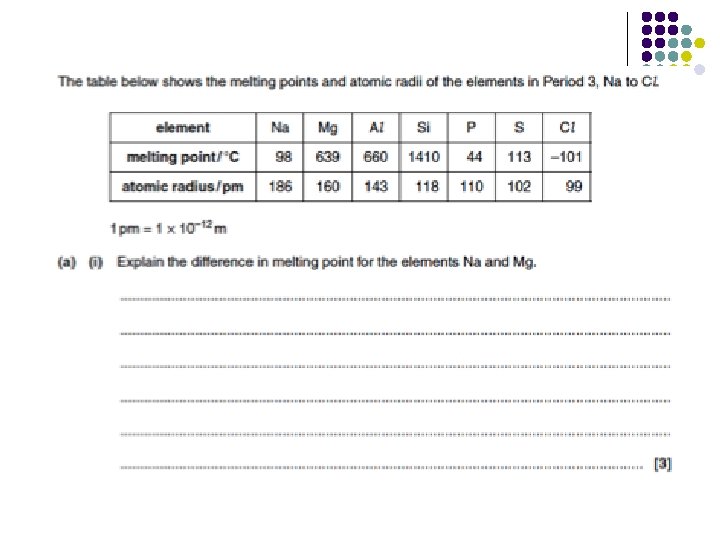
Practise question
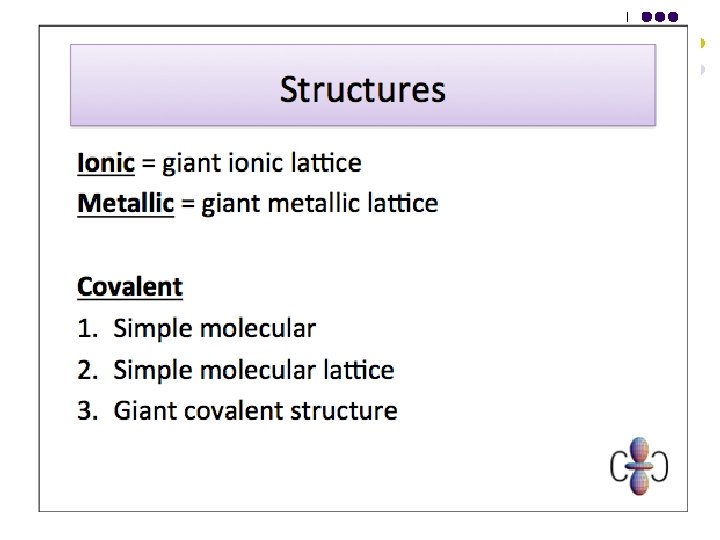
Bonding Structure Covalent bonds are present in two types of molecules - Simple molecular - Giant covalent
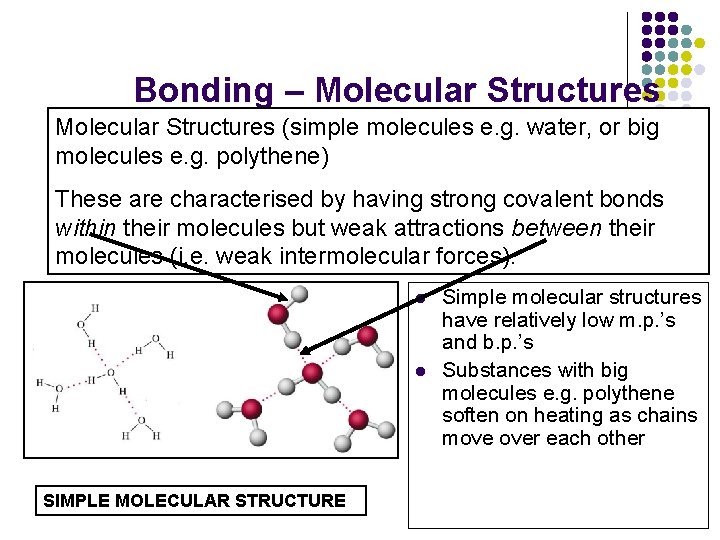
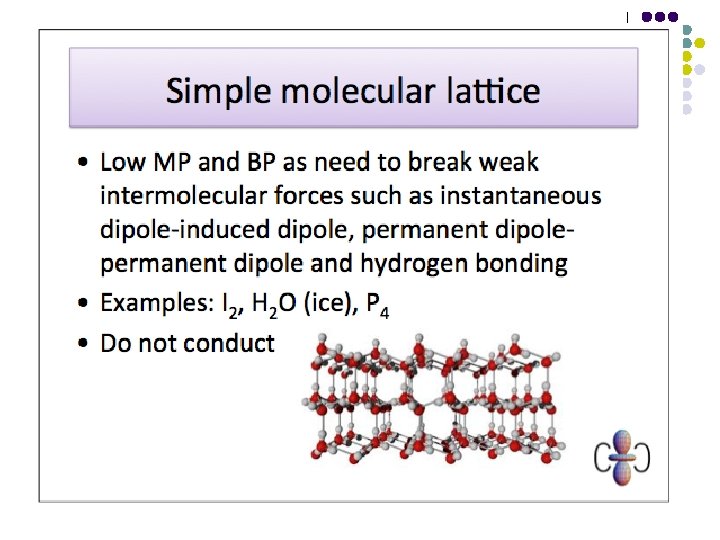
Bonding – Molecular Structures (simple molecules e. g. water, or big molecules e. g. polythene) These are characterised by having strong covalent bonds within their molecules but weak attractions between their molecules (i. e. weak intermolecular forces). l l SIMPLE MOLECULAR STRUCTURE Simple molecular structures have relatively low m. p. ’s and b. p. ’s Substances with big molecules e. g. polythene soften on heating as chains move over each other
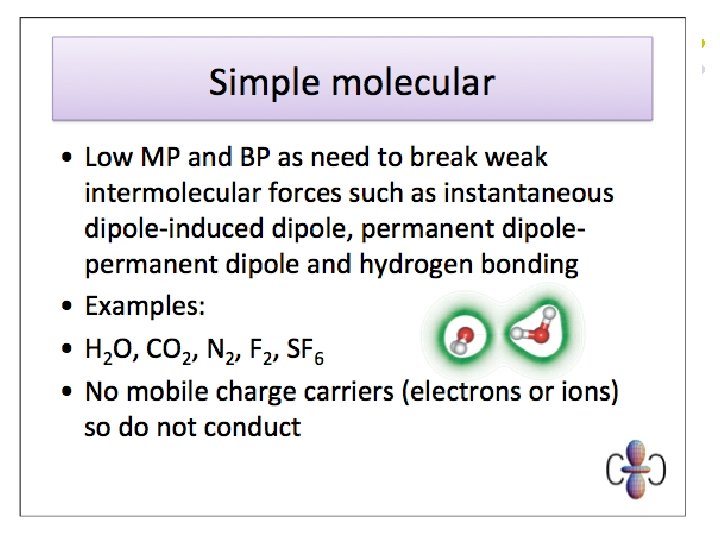
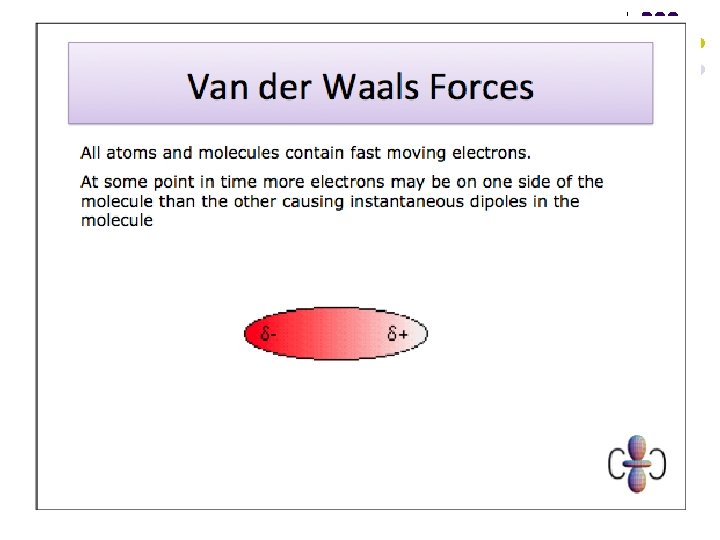
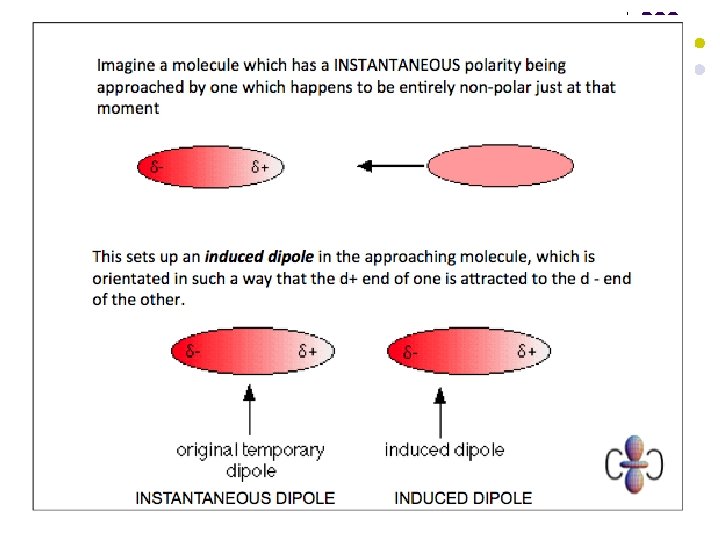

Van der Waals Forces l l These are the weak electrostatic forces of attraction between molecules where one end of a temporary dipole, set up in a molecule as a result of the fluctuating electron cloud, is attracted towards the opposite end of a dipole induced in a neighbouring molecule. Van der Waals forces are the only intermolecular force present in non-polar substances. Van der Waals forces increase in strength with increasing molecular size (i. e. increasing number of electrons per molecule). Van der Waals forces can also be affected by molecular shape – they increase in strength with increasing surface area of contact between molecules.
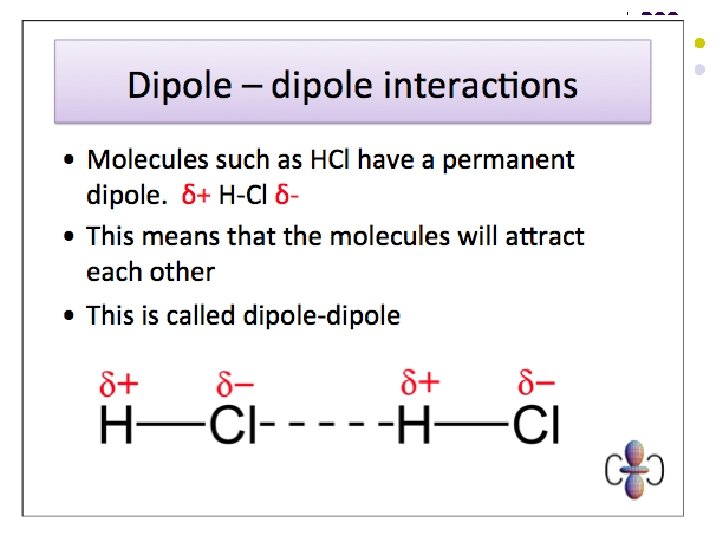

Dipole-dipole attractions l l These are the electrostatic forces of attraction between the oppositely charged ends of permanent dipoles in neighbouring molecules. They exist in addition to Van der Waals forces in polar substances. A covalent bond is polarised if there is a difference in electronegativity between the atoms involved. The molecule as a whole will not be polar if it is symmetrical and the effects of the polarised bonds cancel each other out.
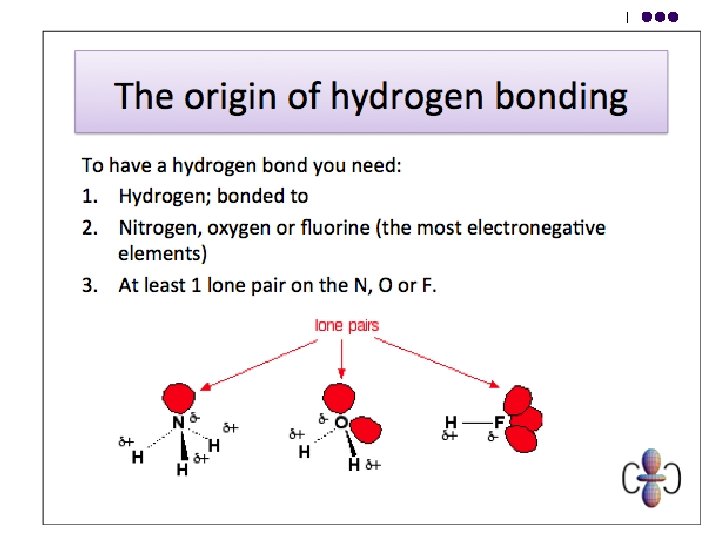

Hydrogen Bonds l l This is the electrostatic force of attraction between a proton that has been denuded of electrons, by direct attachment to a highly electronegative atom such as N, O or F, and the lone pair on another highly electronegative atom. Hydrogen bonding leads to stronger intermolecular forces than ordinary dipole-dipole attractions
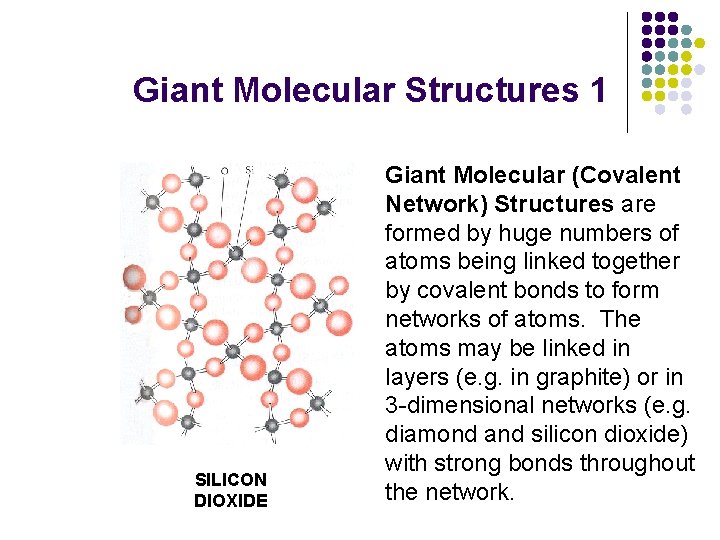
Giant Molecular Structures 1 SILICON DIOXIDE Giant Molecular (Covalent Network) Structures are formed by huge numbers of atoms being linked together by covalent bonds to form networks of atoms. The atoms may be linked in layers (e. g. in graphite) or in 3 -dimensional networks (e. g. diamond and silicon dioxide) with strong bonds throughout the network.
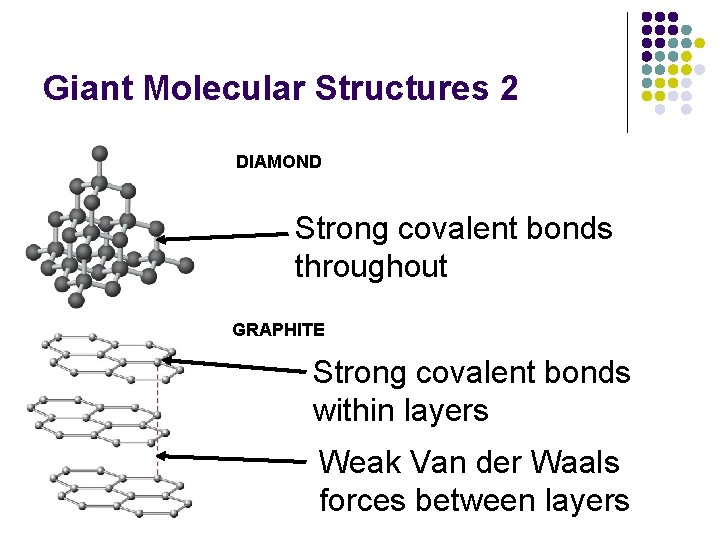
Giant Molecular Structures 2 DIAMOND Strong covalent bonds throughout GRAPHITE Strong covalent bonds within layers Weak Van der Waals forces between layers
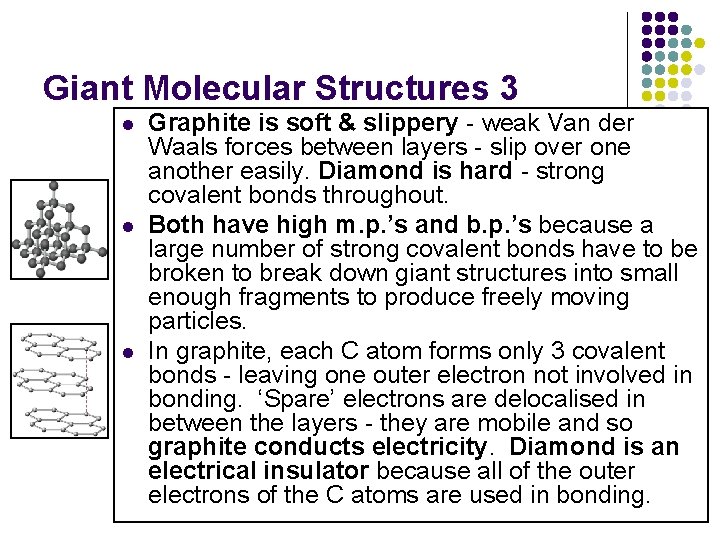
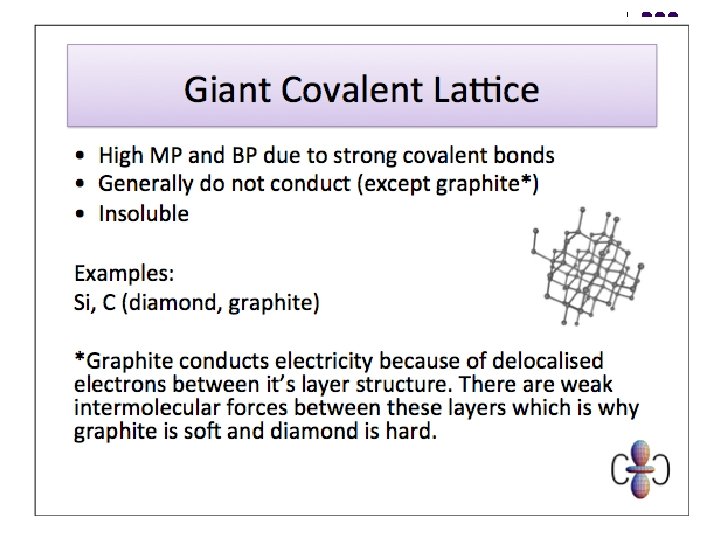
Giant Molecular Structures 3 l l l Graphite is soft & slippery - weak Van der Waals forces between layers - slip over one another easily. Diamond is hard - strong covalent bonds throughout. Both have high m. p. ’s and b. p. ’s because a large number of strong covalent bonds have to be broken to break down giant structures into small enough fragments to produce freely moving particles. In graphite, each C atom forms only 3 covalent bonds - leaving one outer electron not involved in bonding. ‘Spare’ electrons are delocalised in between the layers - they are mobile and so graphite conducts electricity. Diamond is an electrical insulator because all of the outer electrons of the C atoms are used in bonding.

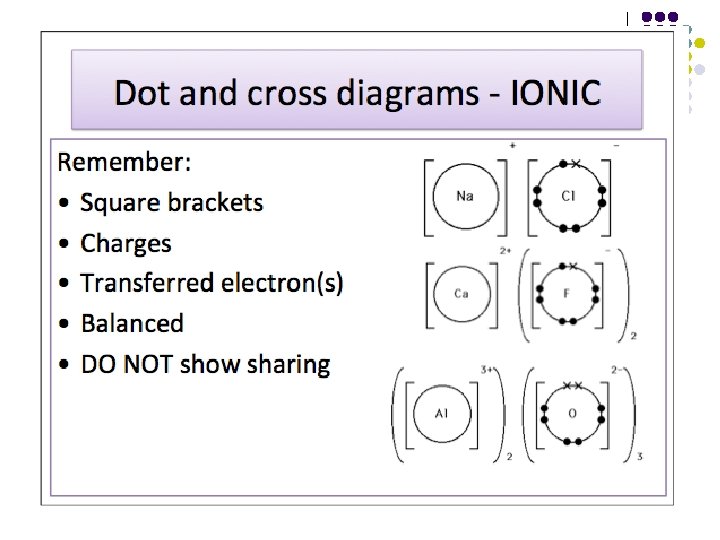
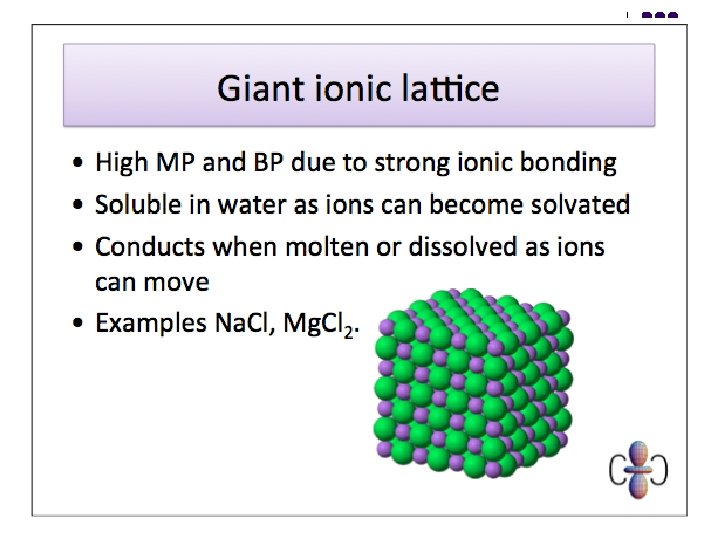
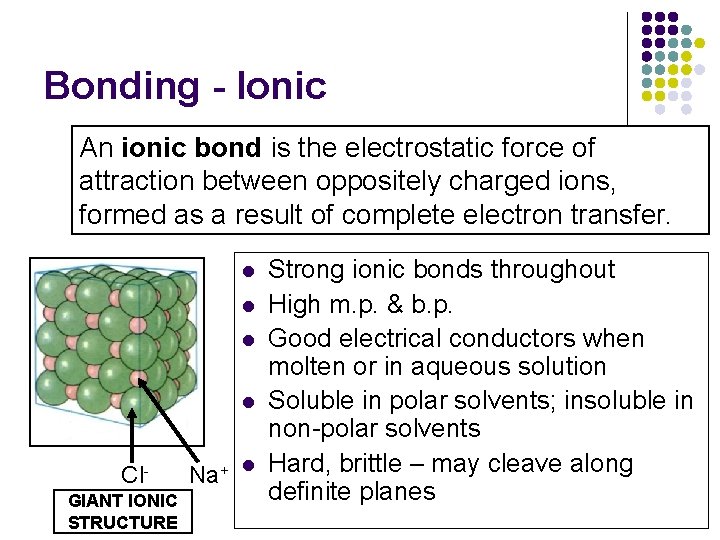
Bonding - Ionic An ionic bond is the electrostatic force of attraction between oppositely charged ions, formed as a result of complete electron transfer. l l Cl. GIANT IONIC STRUCTURE Na+ l Strong ionic bonds throughout High m. p. & b. p. Good electrical conductors when molten or in aqueous solution Soluble in polar solvents; insoluble in non-polar solvents Hard, brittle – may cleave along definite planes
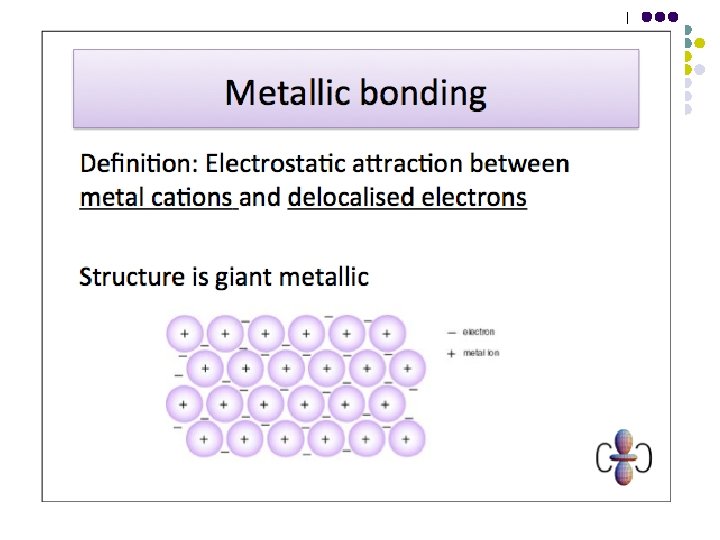
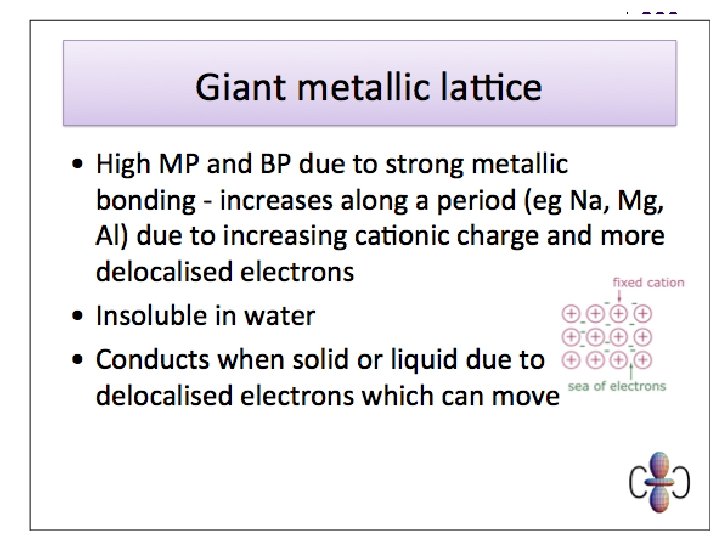
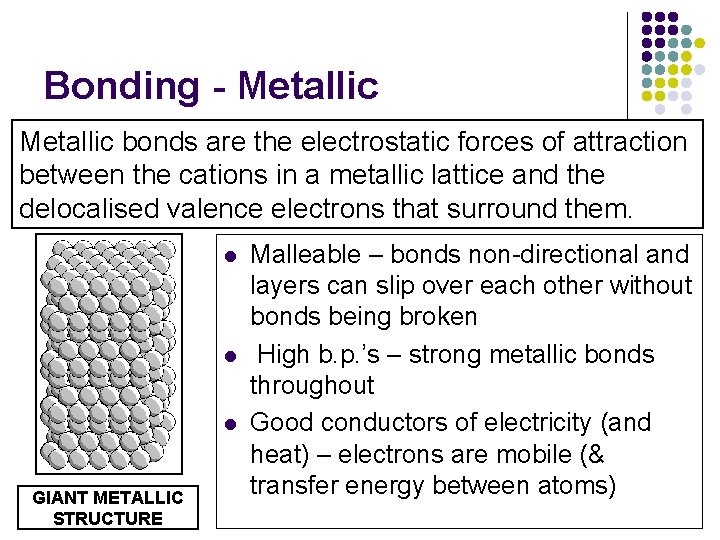
Bonding - Metallic bonds are the electrostatic forces of attraction between the cations in a metallic lattice and the delocalised valence electrons that surround them. l l l GIANT METALLIC STRUCTURE Malleable – bonds non-directional and layers can slip over each other without bonds being broken High b. p. ’s – strong metallic bonds throughout Good conductors of electricity (and heat) – electrons are mobile (& transfer energy between atoms)



- Slides: 34