Structure and Bonding Molecular Orbital Theory Hybridization Isomerization
Structure and Bonding Molecular Orbital Theory Hybridization Isomerization Dipoles and Polarity
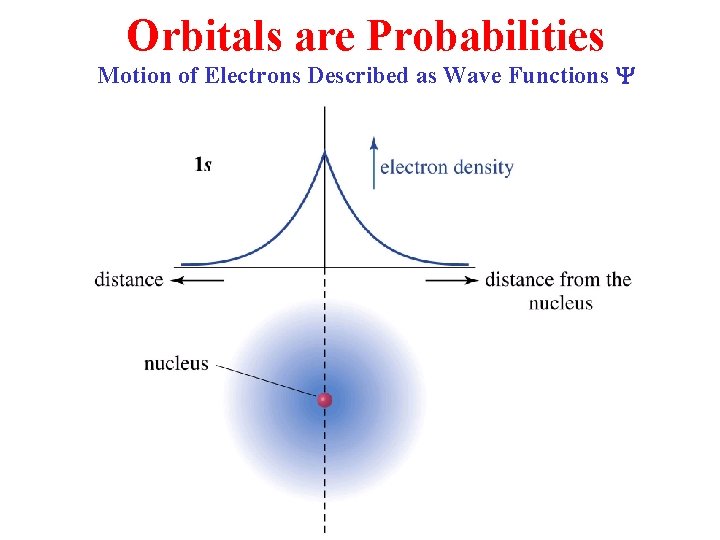
Orbitals are Probabilities Motion of Electrons Described as Wave Functions Y

2 s Orbital Has a Node
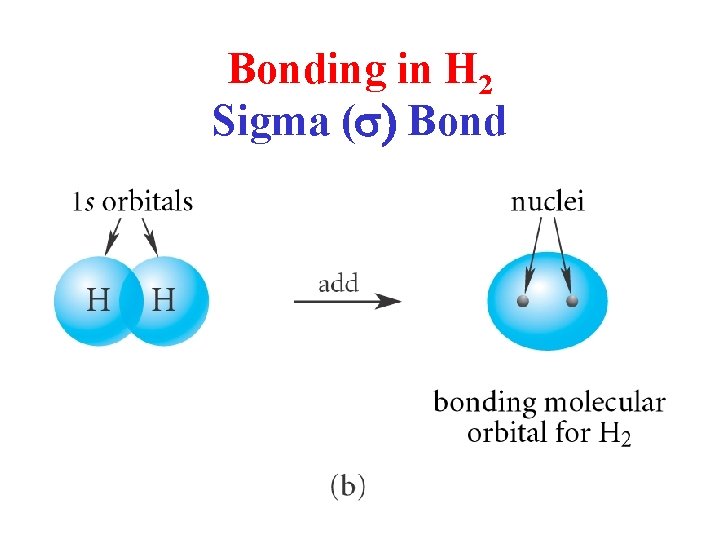
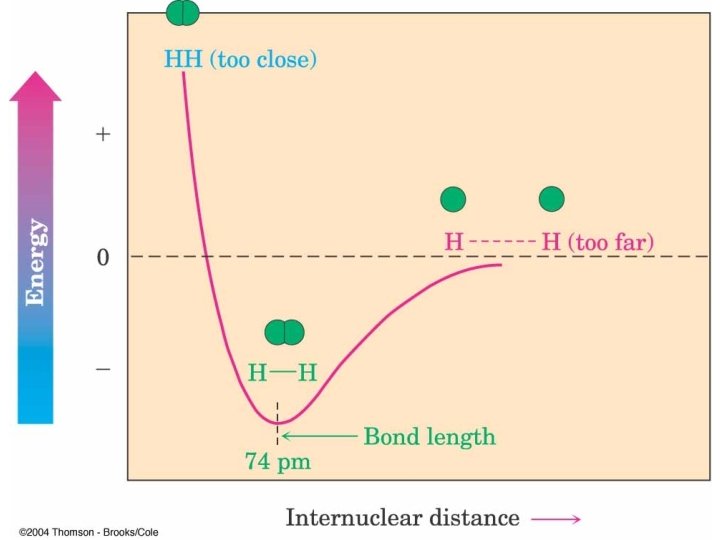
Bonding in H 2 Sigma (s) Bond
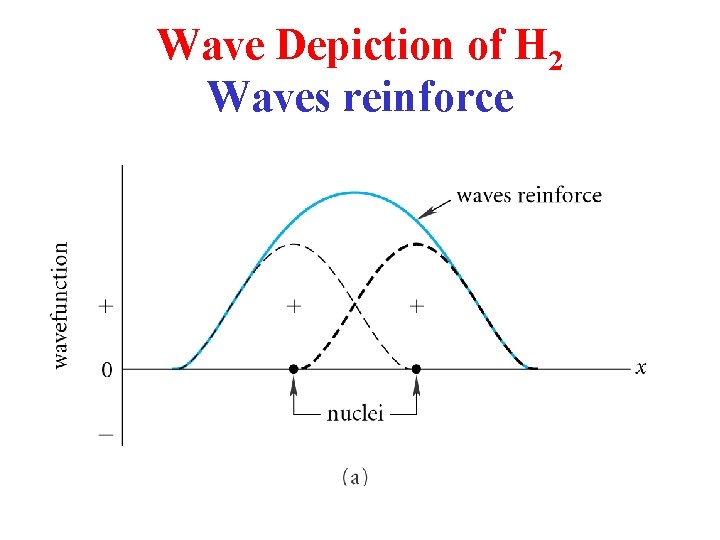
Wave Depiction of H 2 Waves reinforce
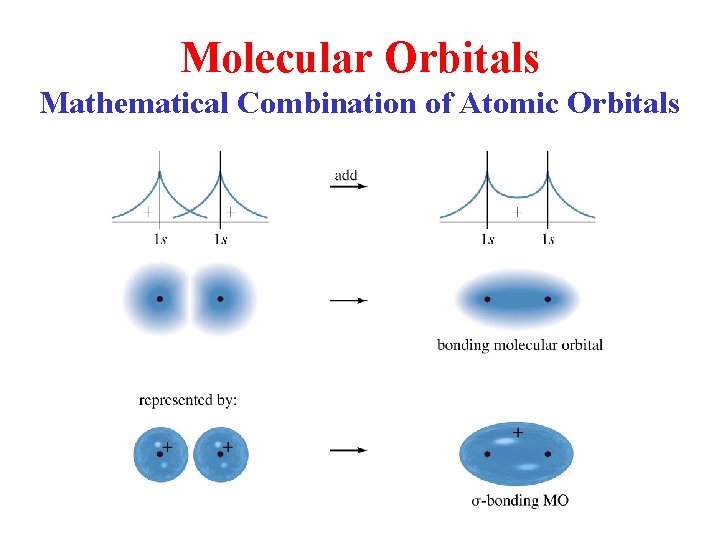
Molecular Orbitals Mathematical Combination of Atomic Orbitals
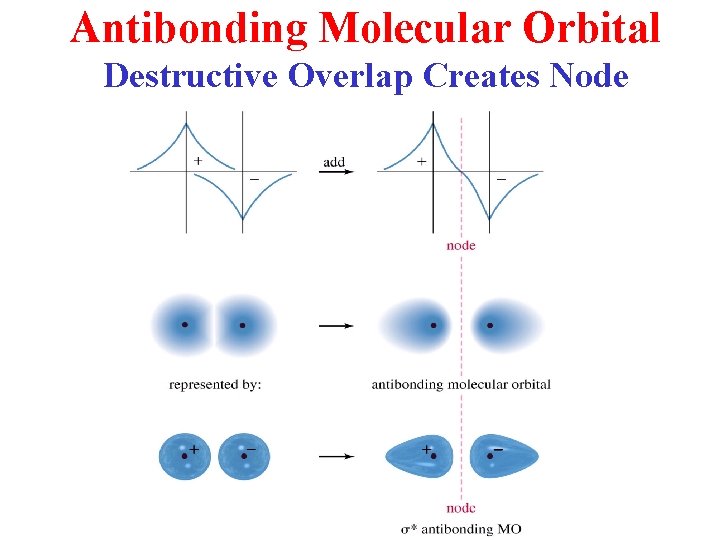
Antibonding Molecular Orbital Destructive Overlap Creates Node
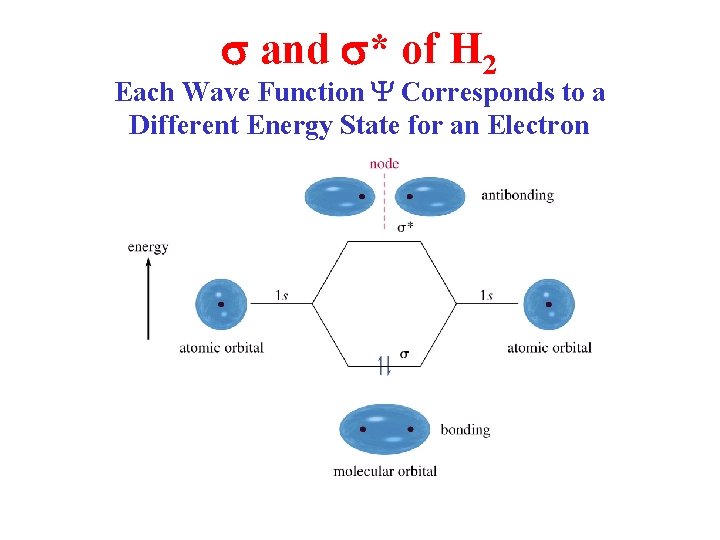
s and s* of H 2 Each Wave Function Y Corresponds to a Different Energy State for an Electron
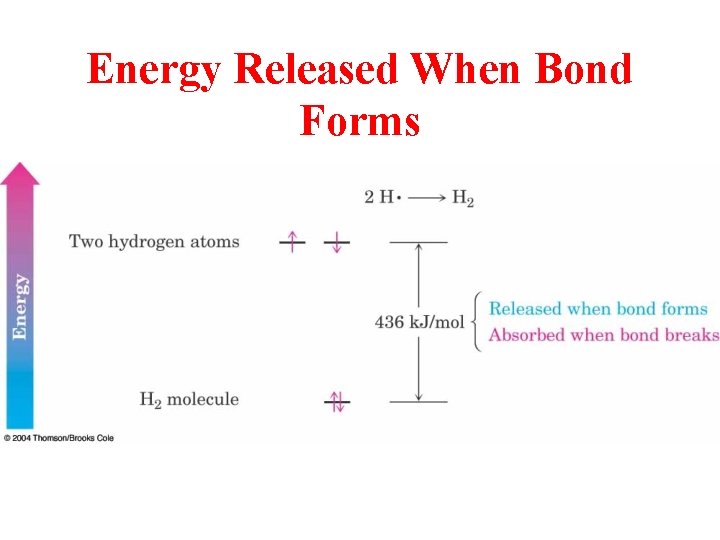
Energy Released When Bond Forms
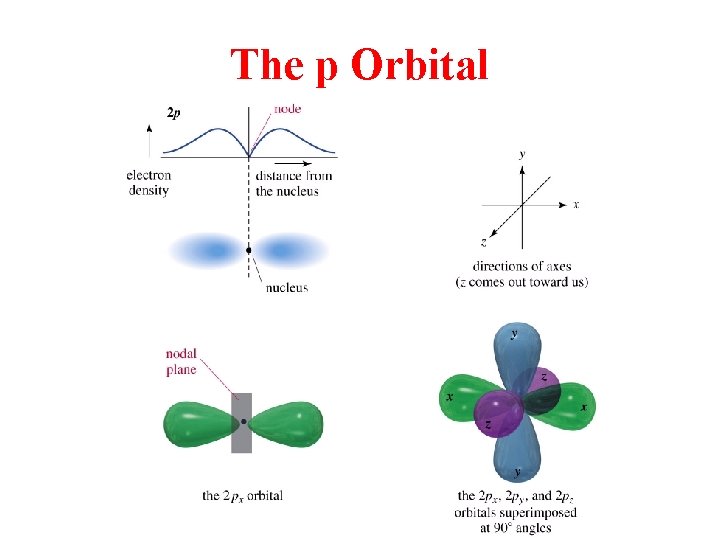
The p Orbital
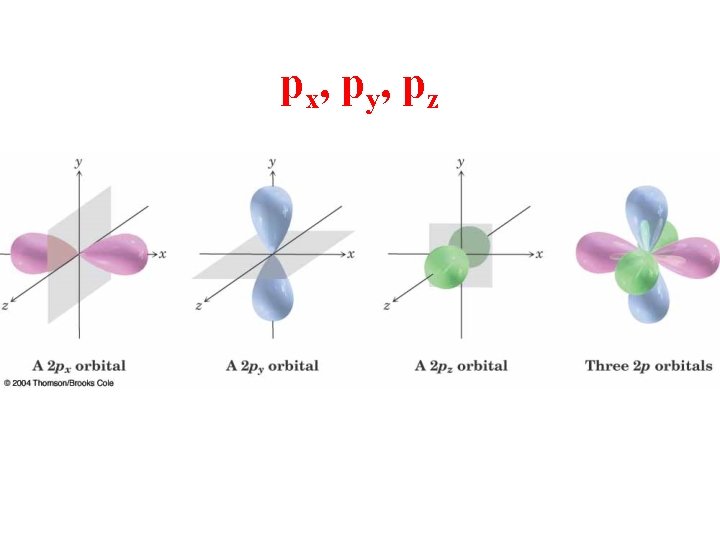
px, py, pz

Ground State Electron Configurations
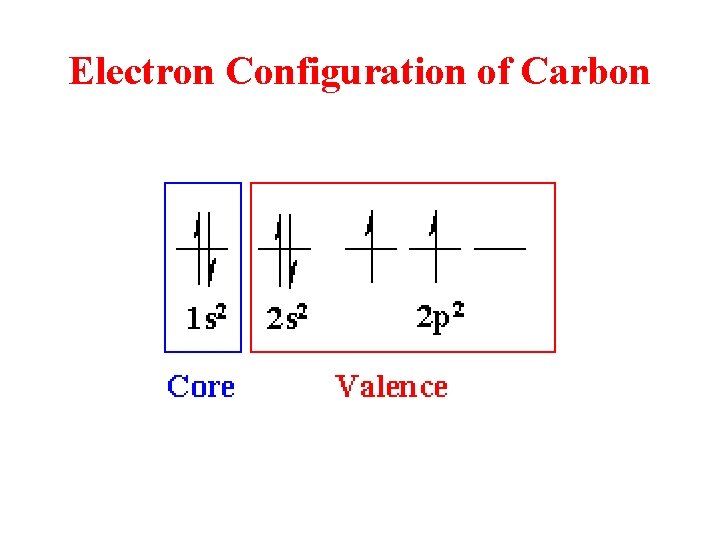
Electron Configuration of Carbon
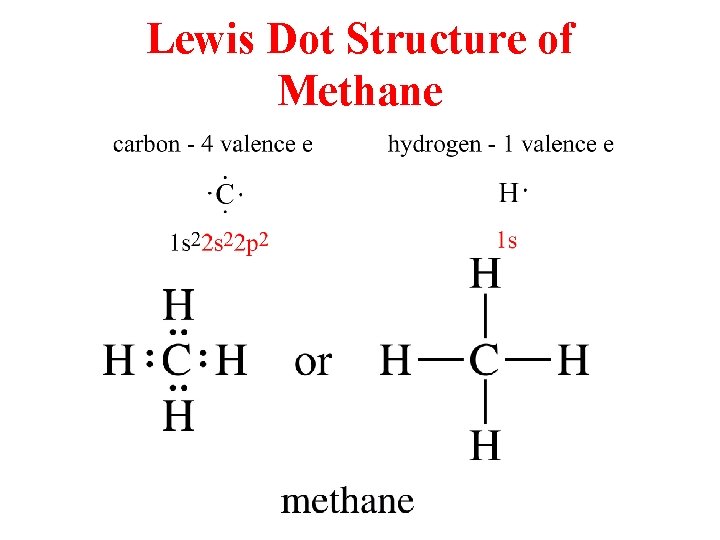
Lewis Dot Structure of Methane
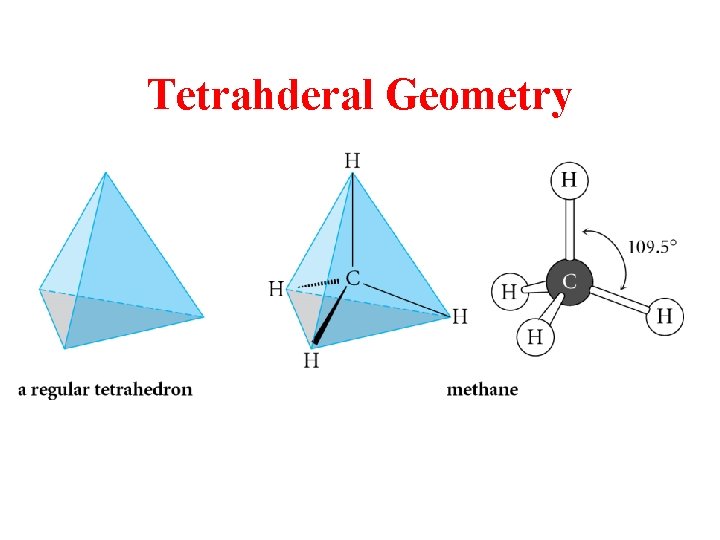
Tetrahderal Geometry

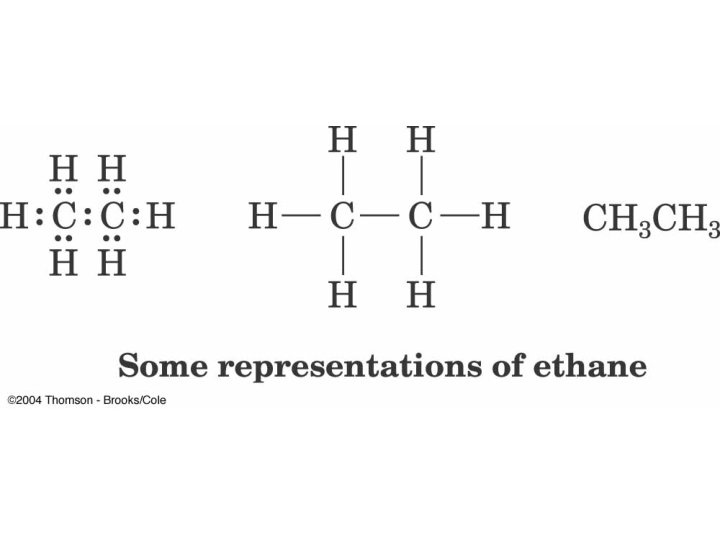
Methane Representations
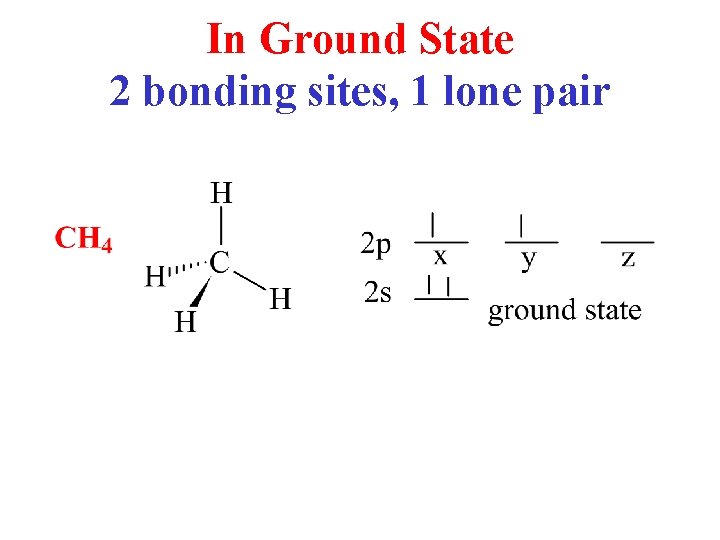
In Ground State 2 bonding sites, 1 lone pair
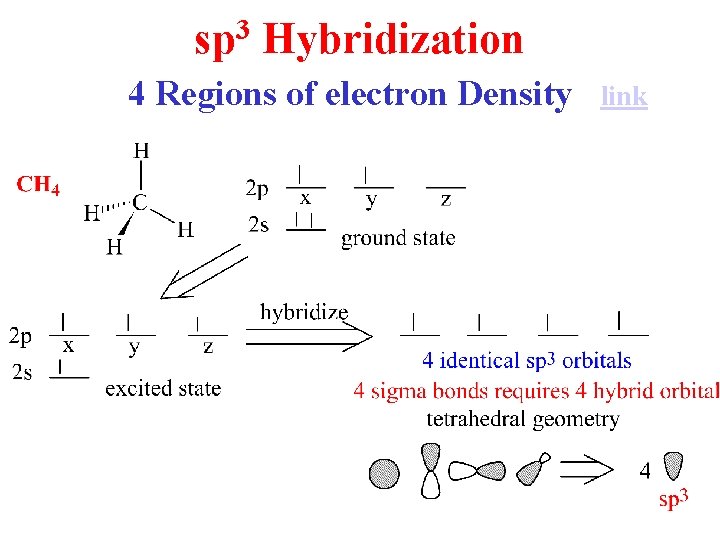
3 sp Hybridization 4 Regions of electron Density link
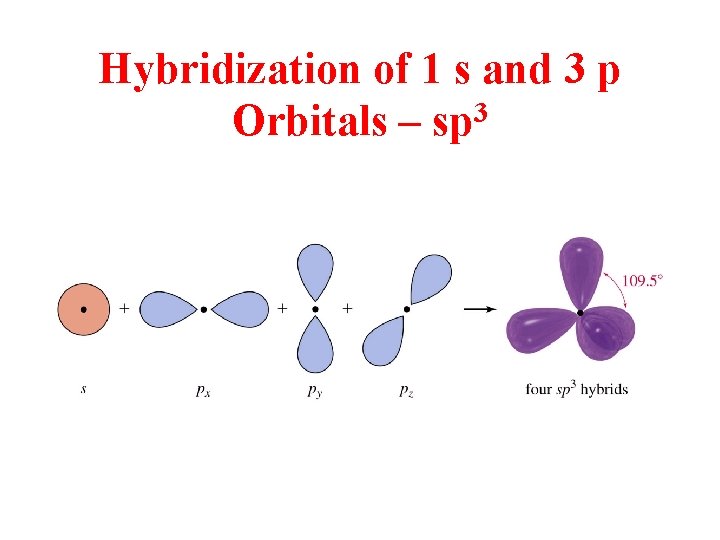
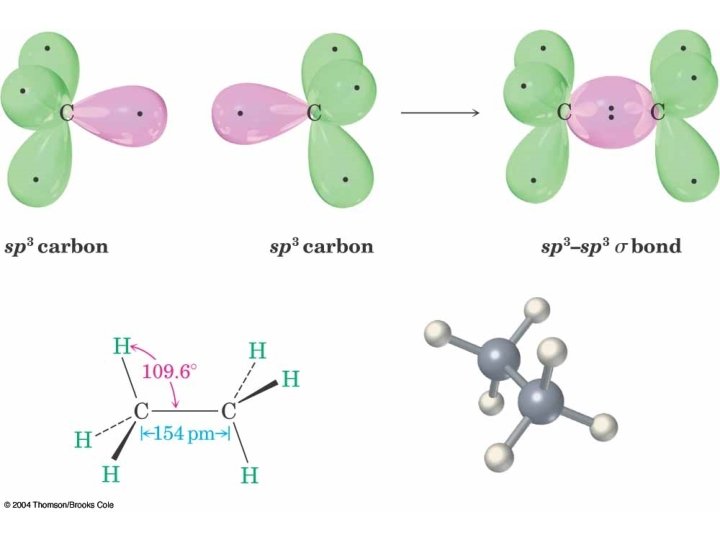
Hybridization of 1 s and 3 p Orbitals – sp 3
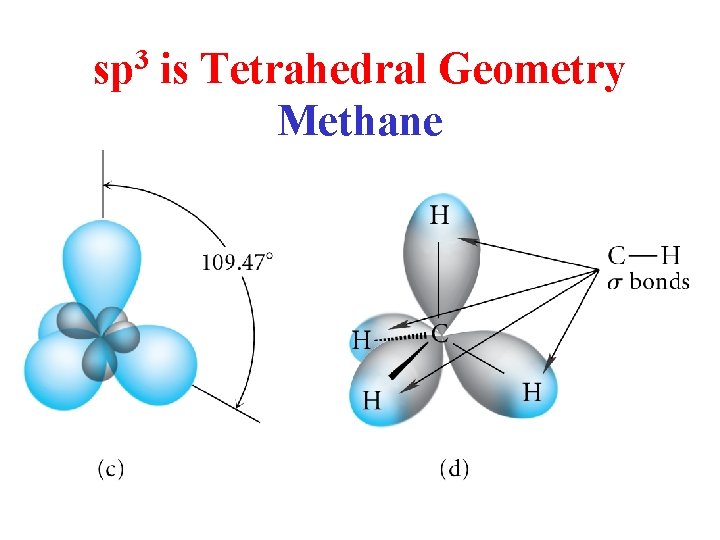
sp 3 is Tetrahedral Geometry Methane
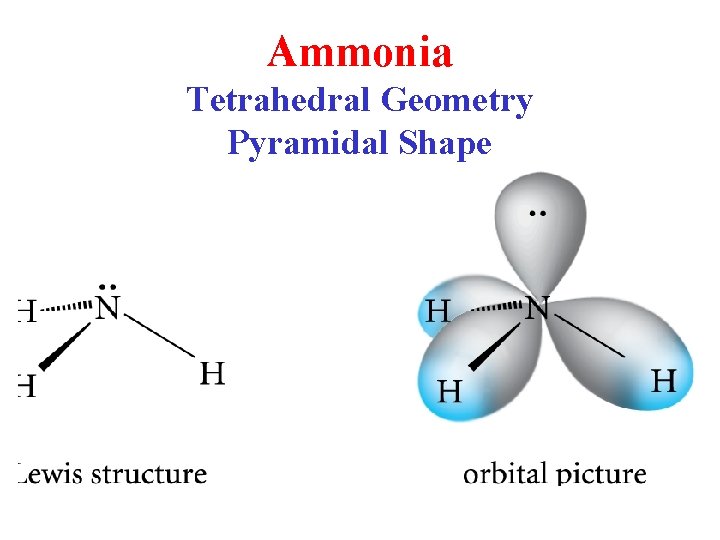
Ammonia Tetrahedral Geometry Pyramidal Shape
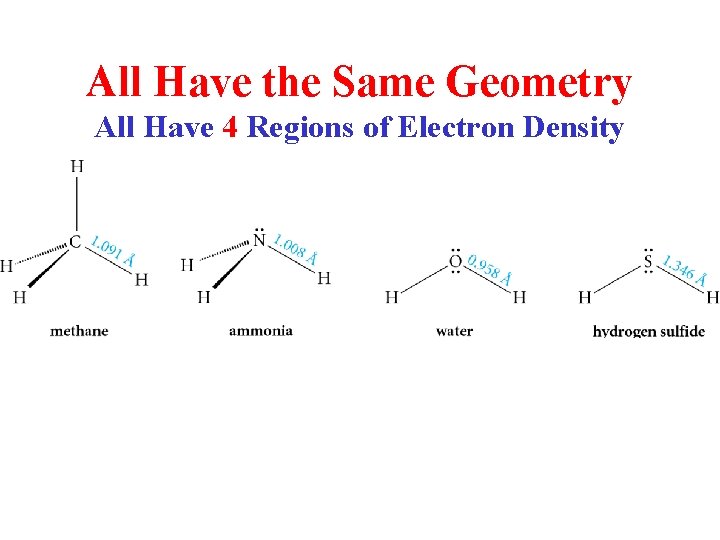
All Have the Same Geometry All Have 4 Regions of Electron Density
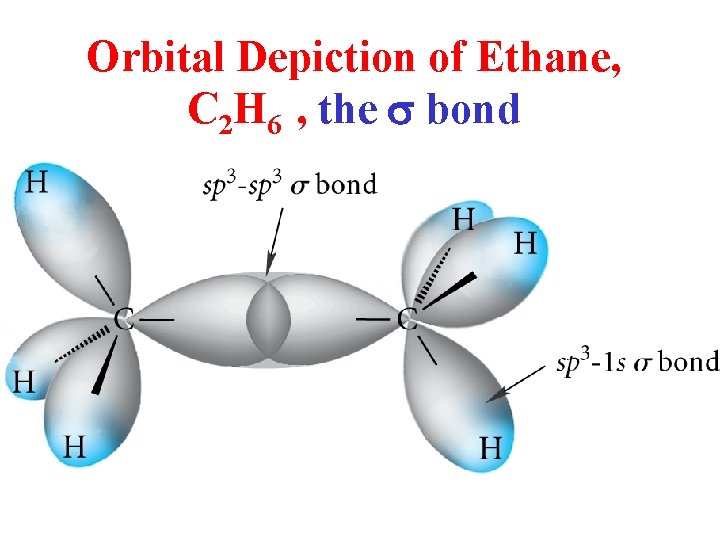
Orbital Depiction of Ethane, C 2 H 6 , the s bond
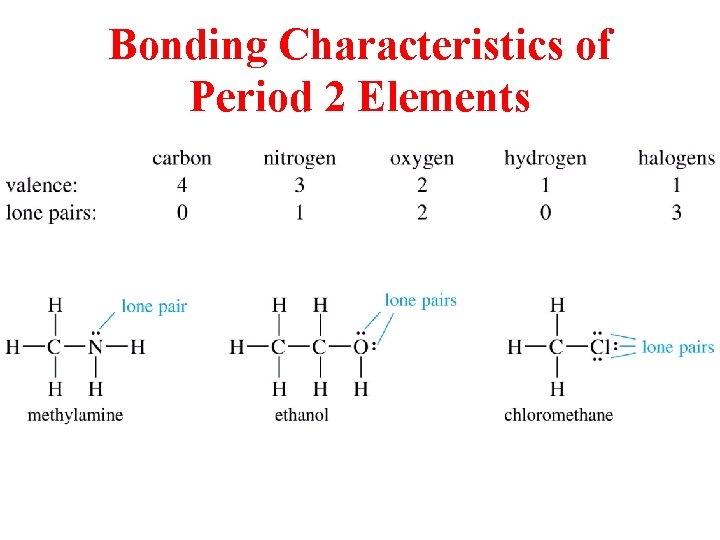
Bonding Characteristics of Period 2 Elements
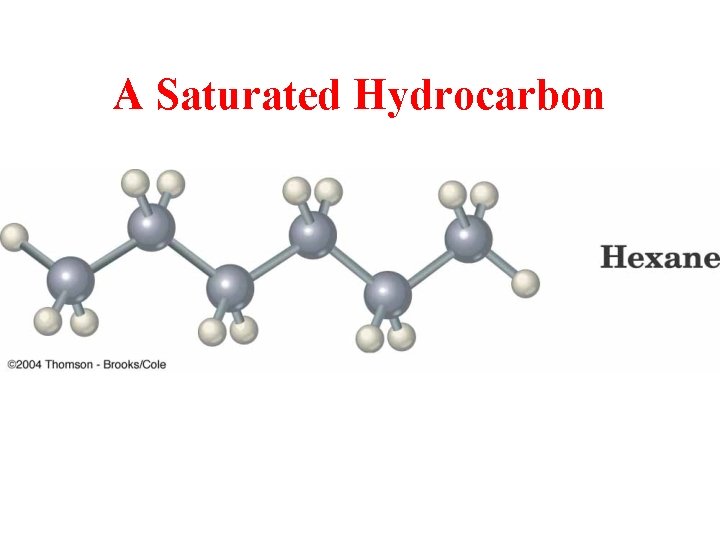
A Saturated Hydrocarbon
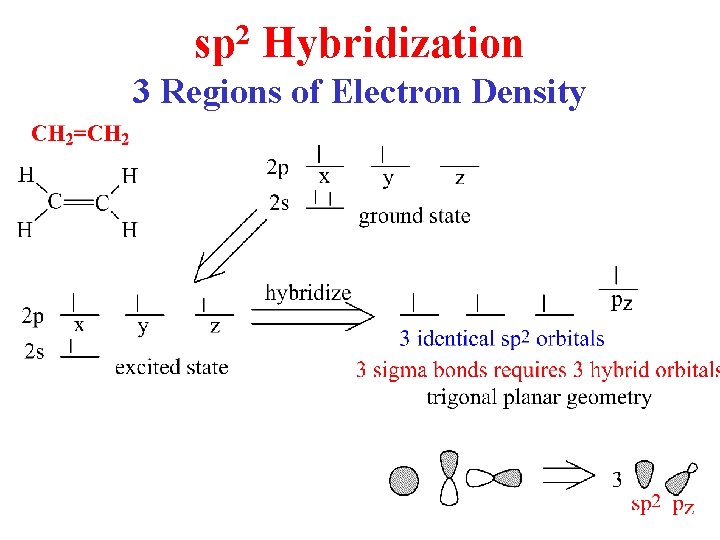
sp 2 Hybridization 3 Regions of Electron Density
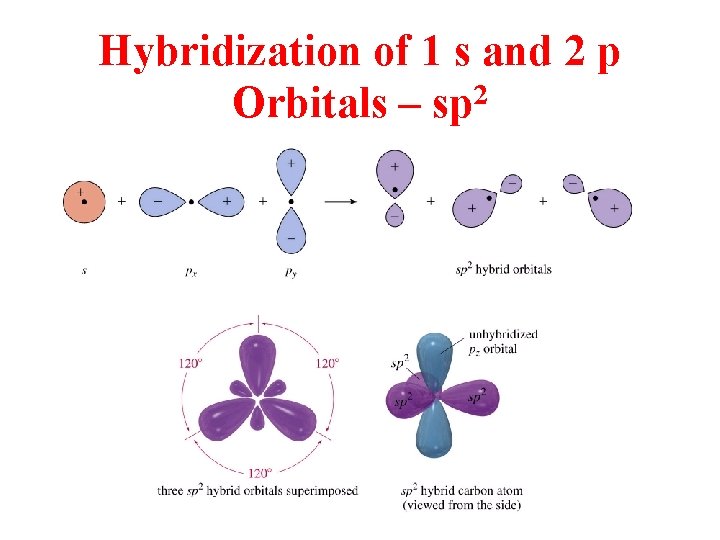
Hybridization of 1 s and 2 p Orbitals – sp 2
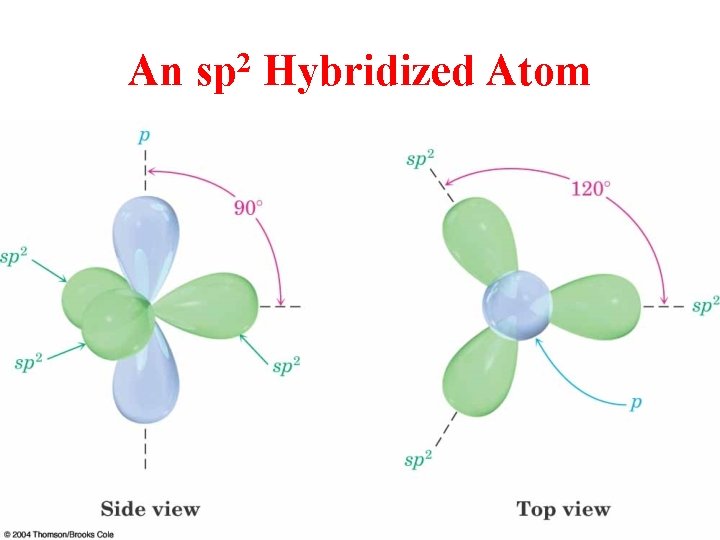
An 2 sp Hybridized Atom
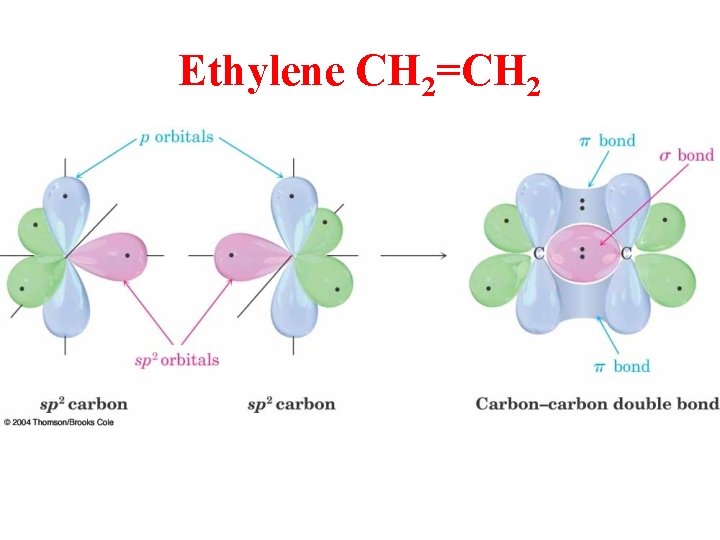
Ethylene CH 2=CH 2
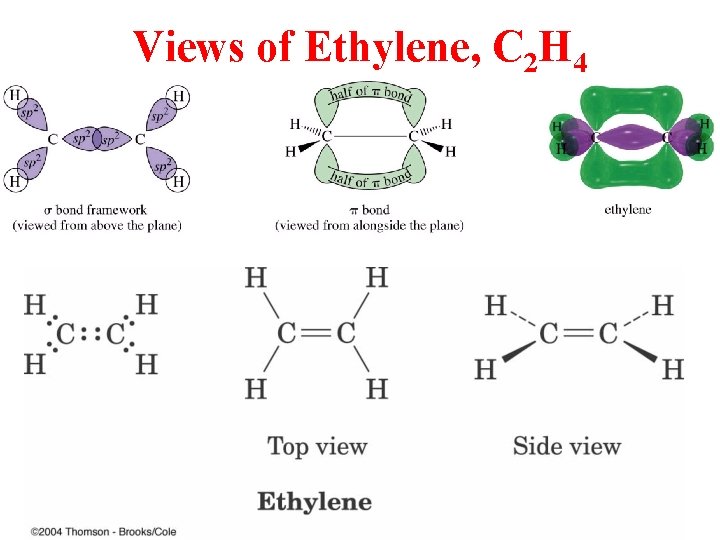
Views of Ethylene, C 2 H 4
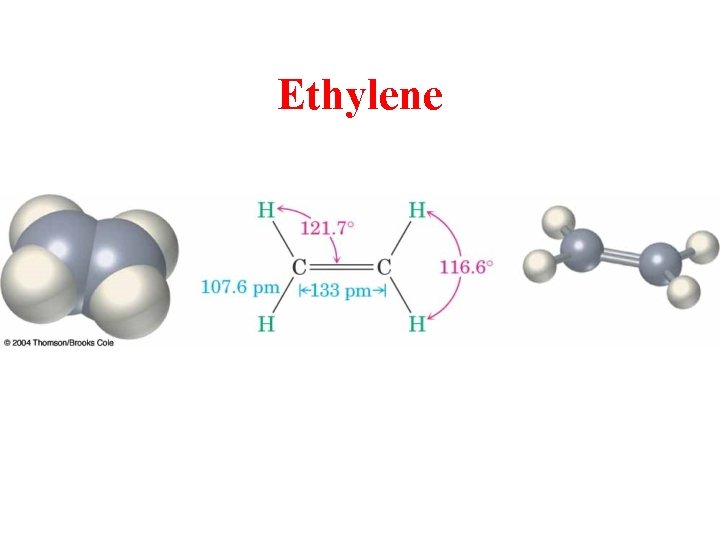
Ethylene

Formaldehyde

sp Hybridization 2 Regions of Electron Density

The sp Orbital
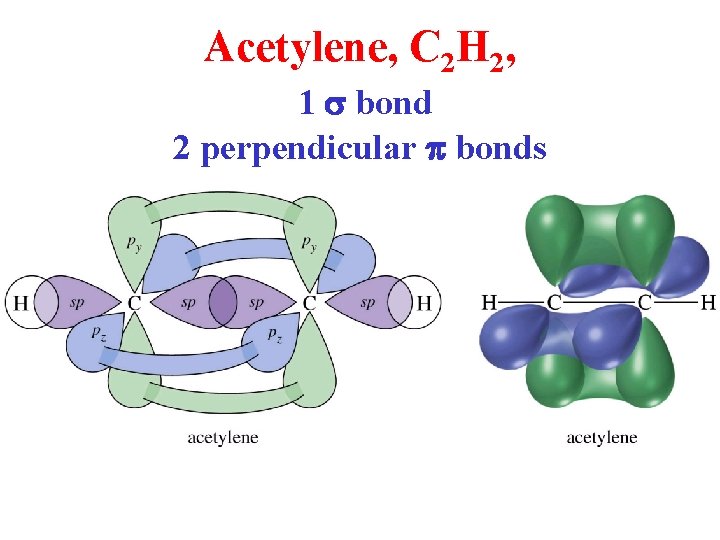
Acetylene, C 2 H 2, 1 s bond 2 perpendicular p bonds
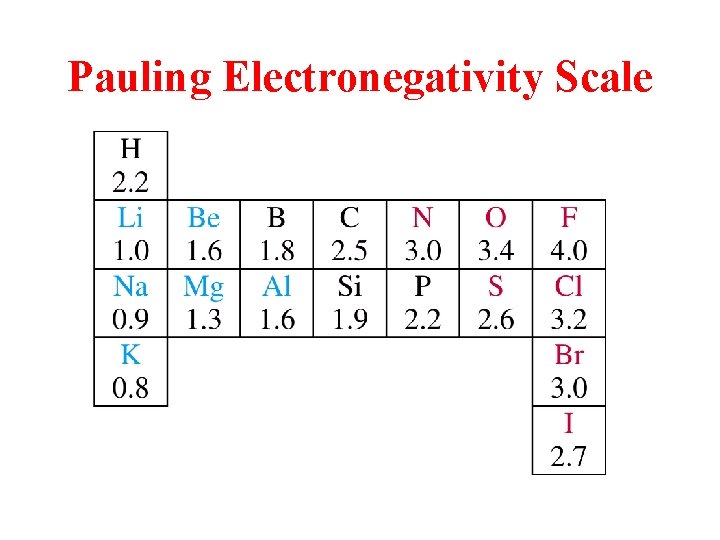
Pauling Electronegativity Scale
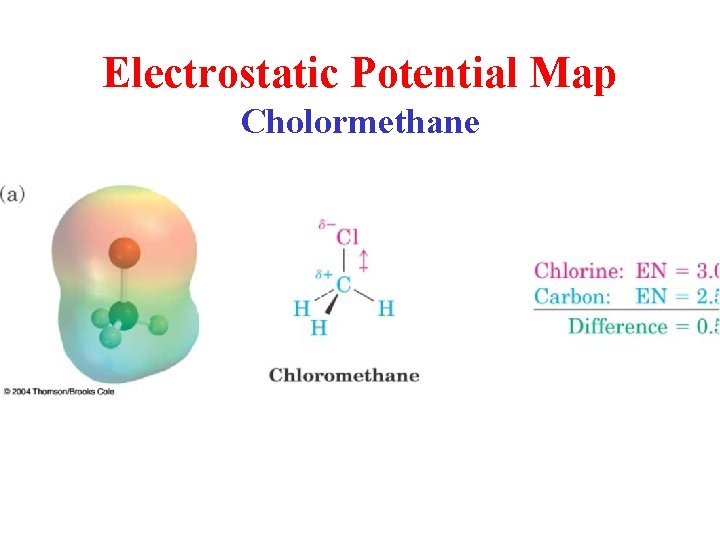
Electrostatic Potential Map Cholormethane
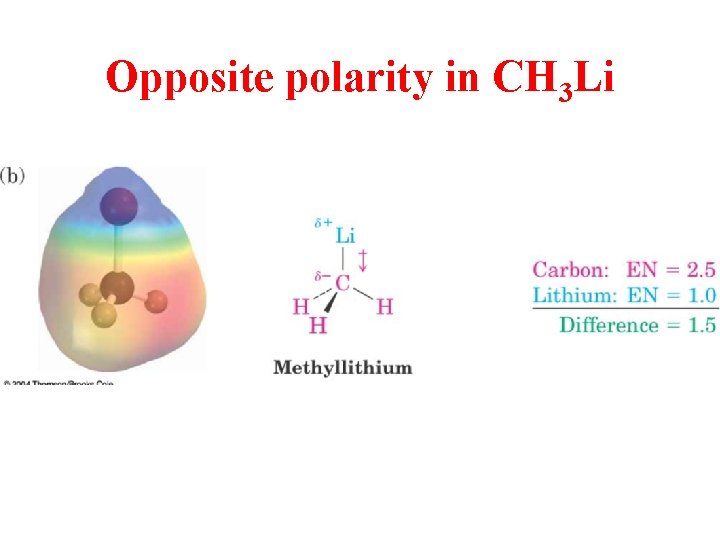
Opposite polarity in CH 3 Li
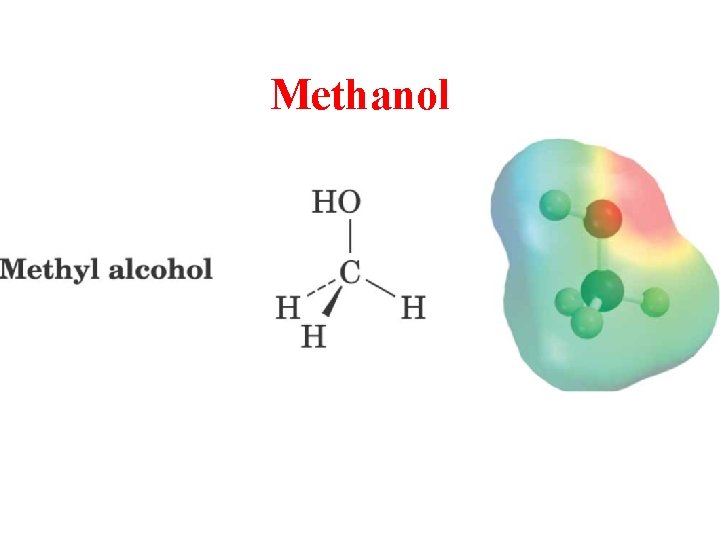
Methanol
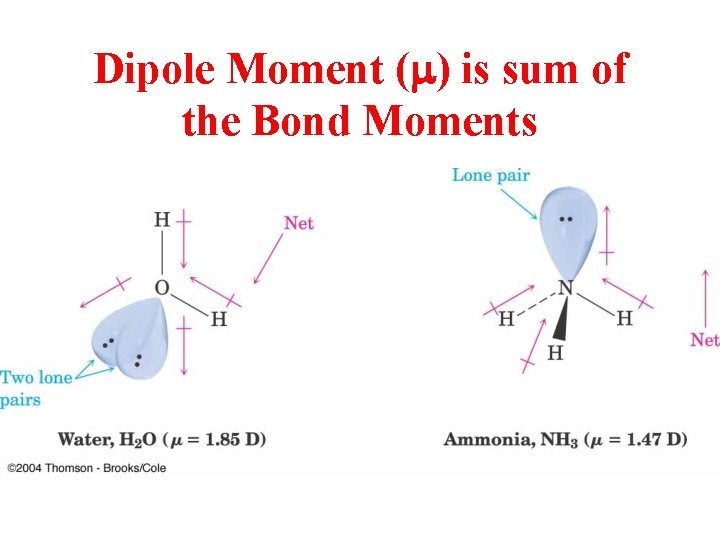
Dipole Moment (m) is sum of the Bond Moments

Nonpolar Compounds Bond Moments Cancel Out

Dipole Moment reflects Both Resonance Structures

NCl 3 and BCl 3

Dipole Moments

Dipole-Dipole Interactions
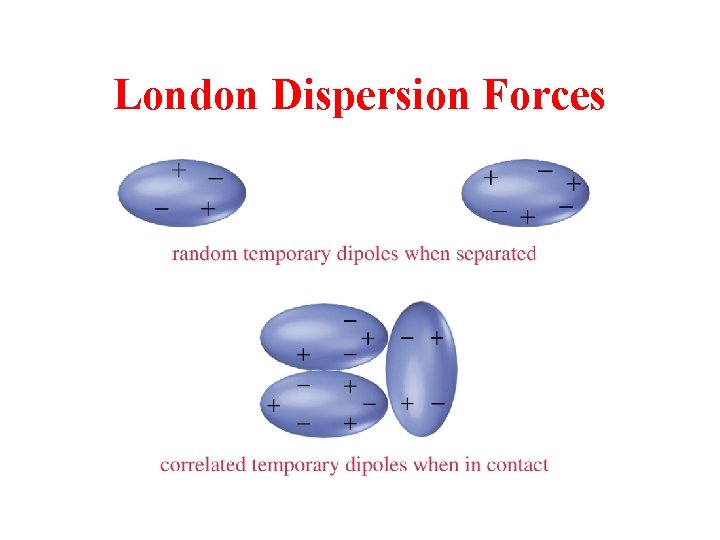
London Dispersion Forces
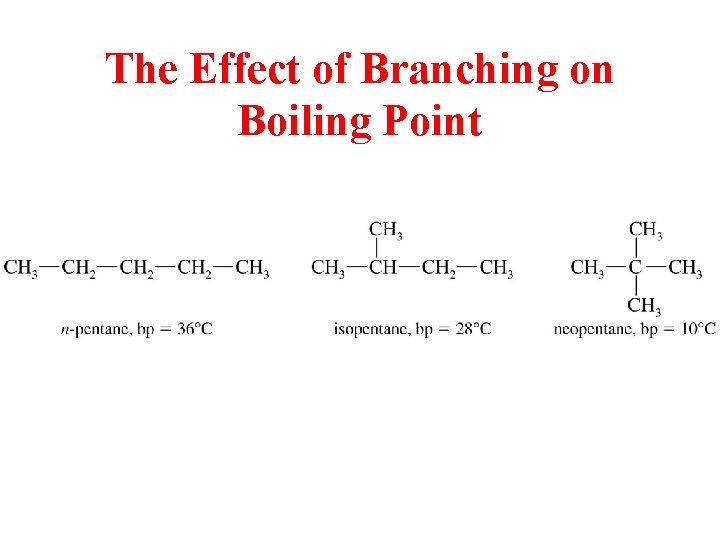
The Effect of Branching on Boiling Point
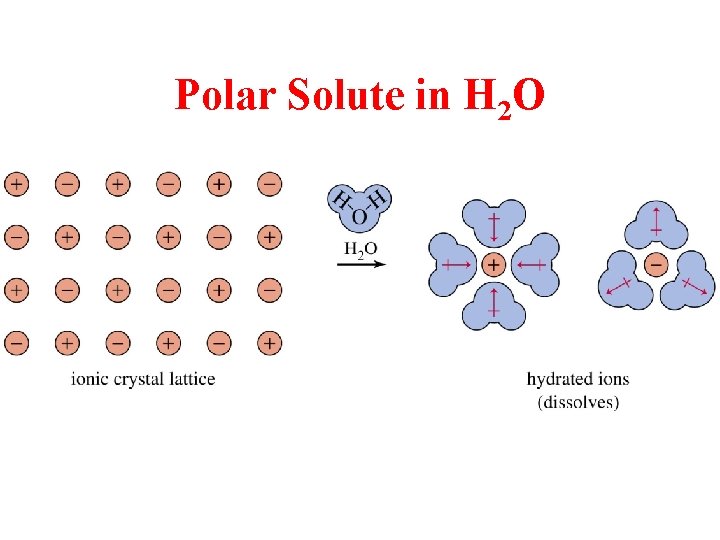
Polar Solute in H 2 O
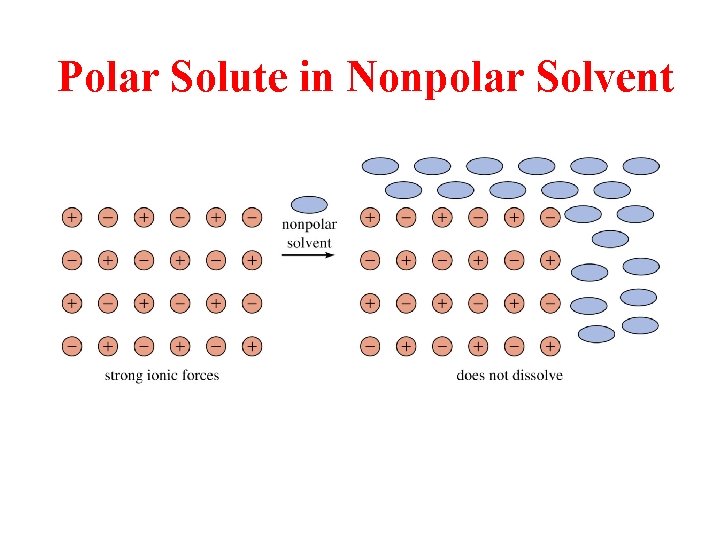
Polar Solute in Nonpolar Solvent
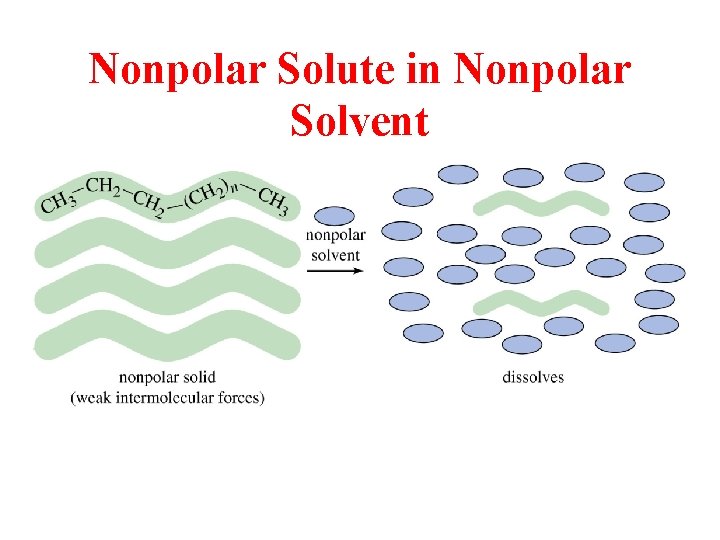
Nonpolar Solute in Nonpolar Solvent
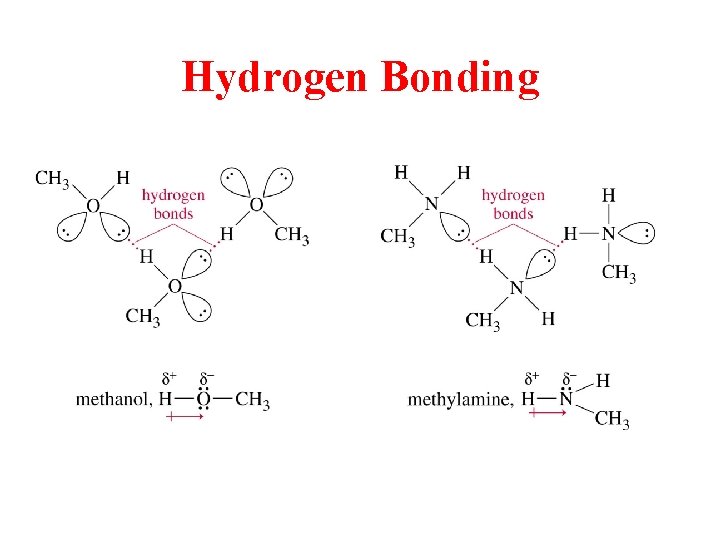
Hydrogen Bonding
- Slides: 55