Structure and Bonding in Organic Chemistry Electron Configurations
Structure and Bonding in Organic Chemistry Electron Configurations Lewis Dot Structures Hybridization Orbital depictions
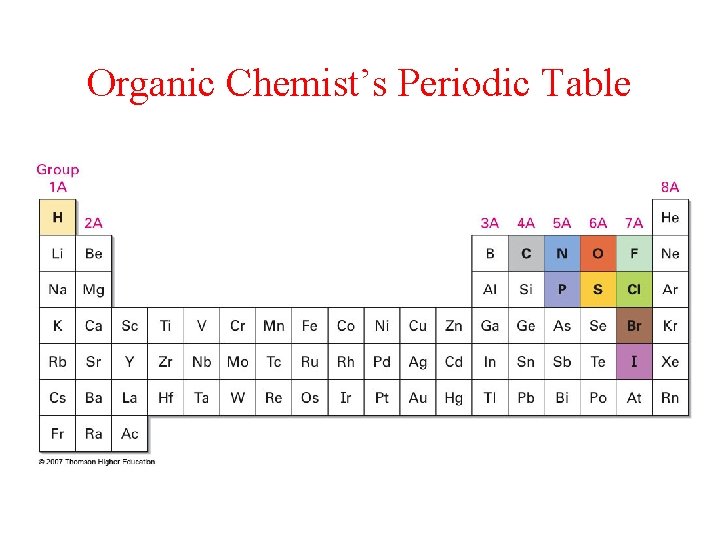
Organic Chemist’s Periodic Table
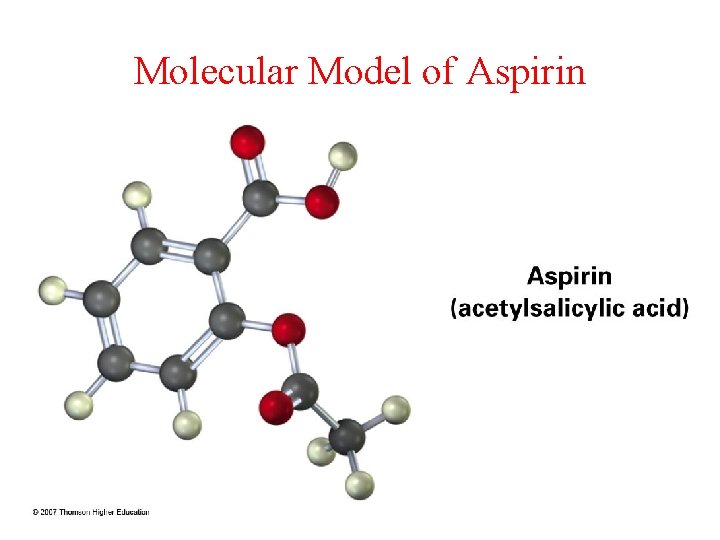
Molecular Model of Aspirin
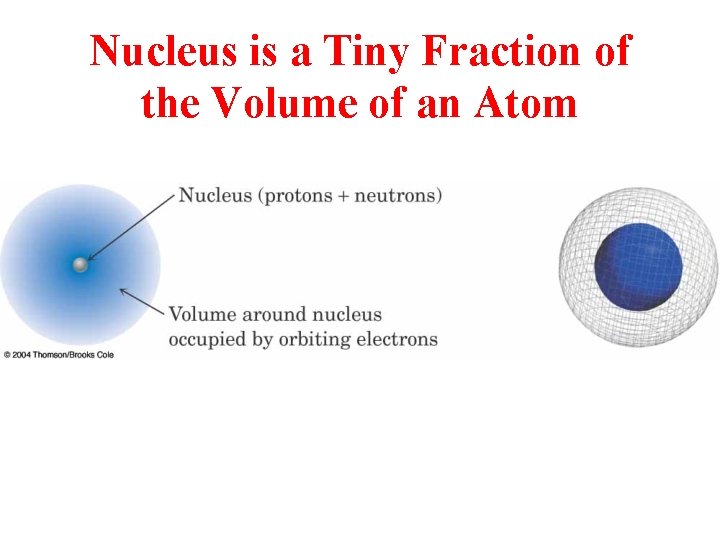
Nucleus is a Tiny Fraction of the Volume of an Atom
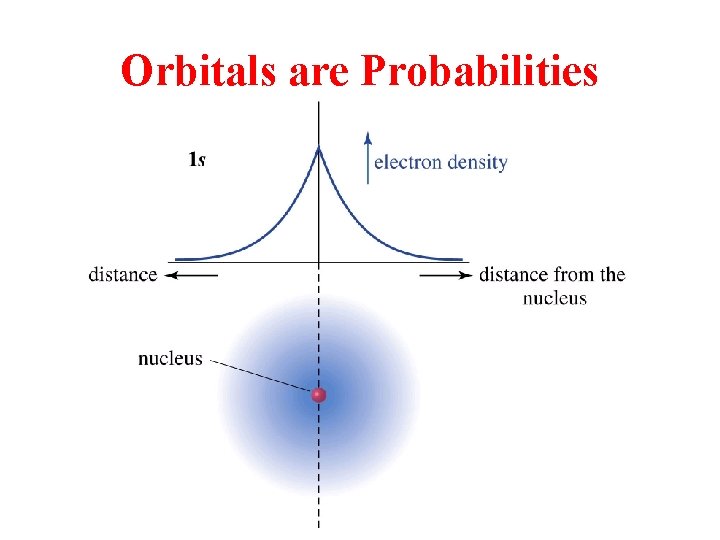
Orbitals are Probabilities
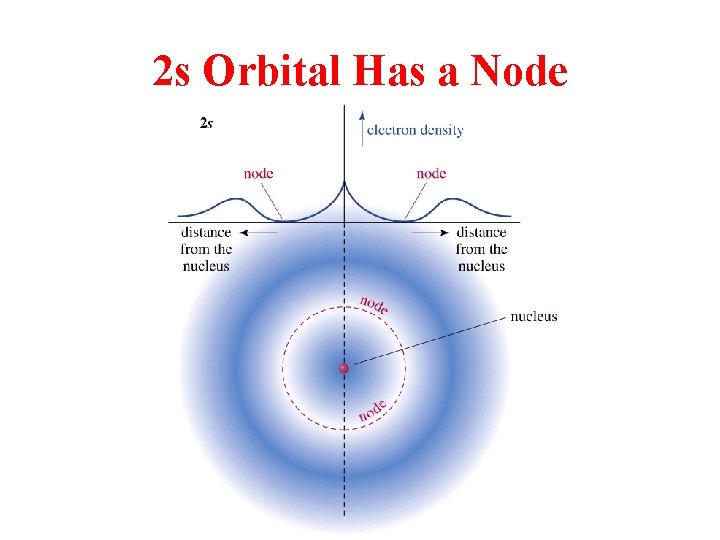
2 s Orbital Has a Node
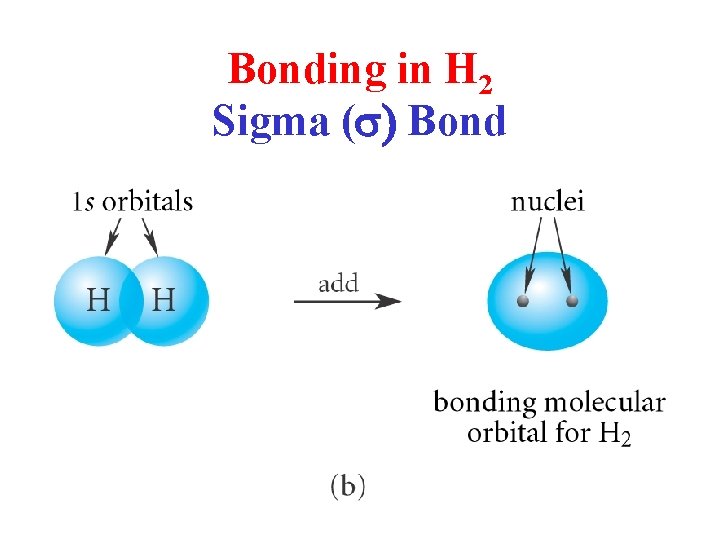
Bonding in H 2 Sigma (s) Bond
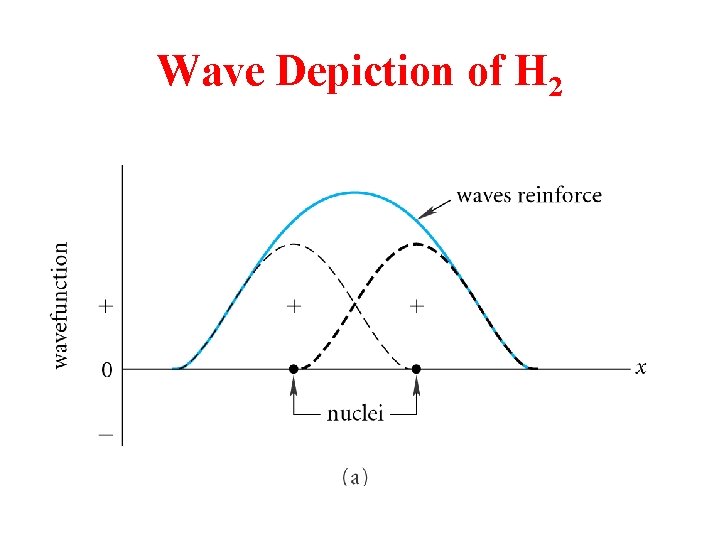
Wave Depiction of H 2
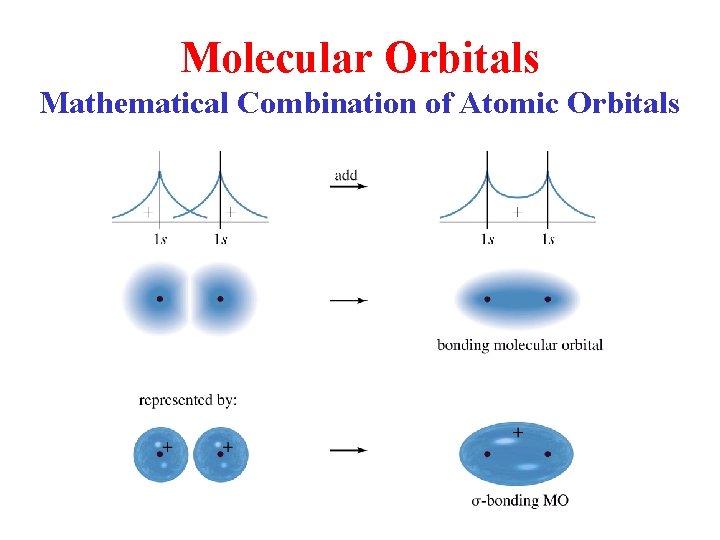
Molecular Orbitals Mathematical Combination of Atomic Orbitals
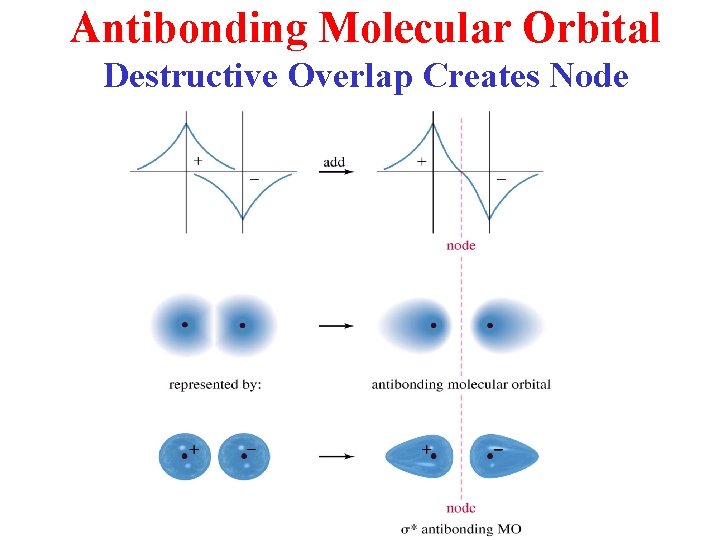
Antibonding Molecular Orbital Destructive Overlap Creates Node
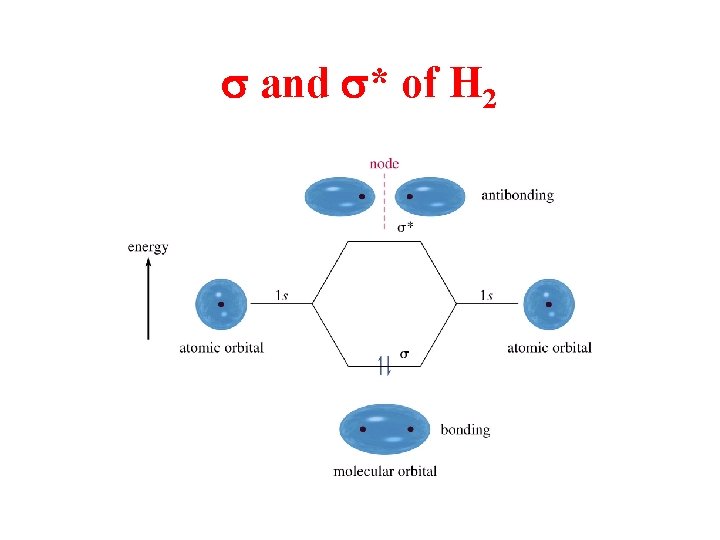
s and s* of H 2
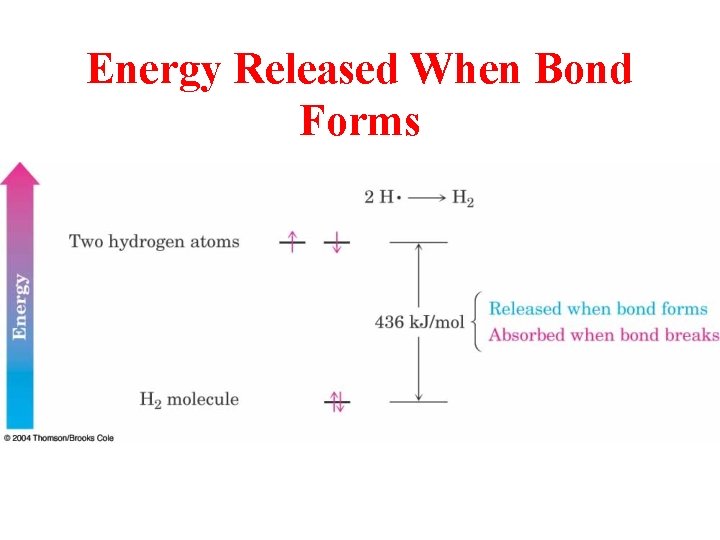
Energy Released When Bond Forms
Electron Configurations
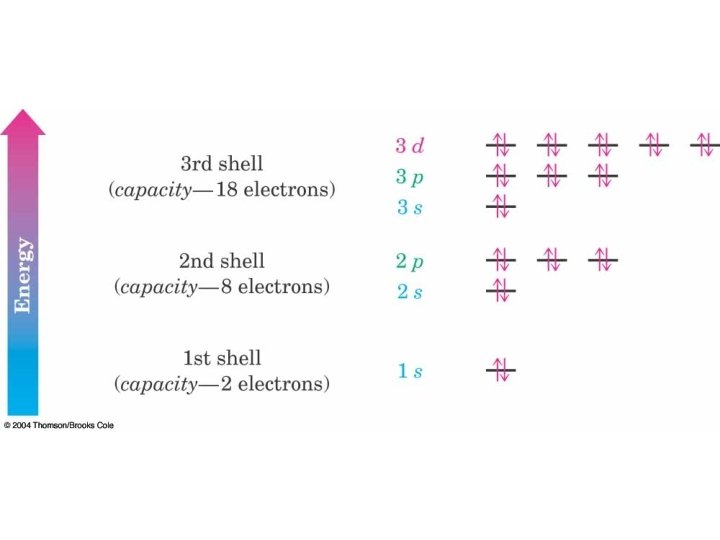
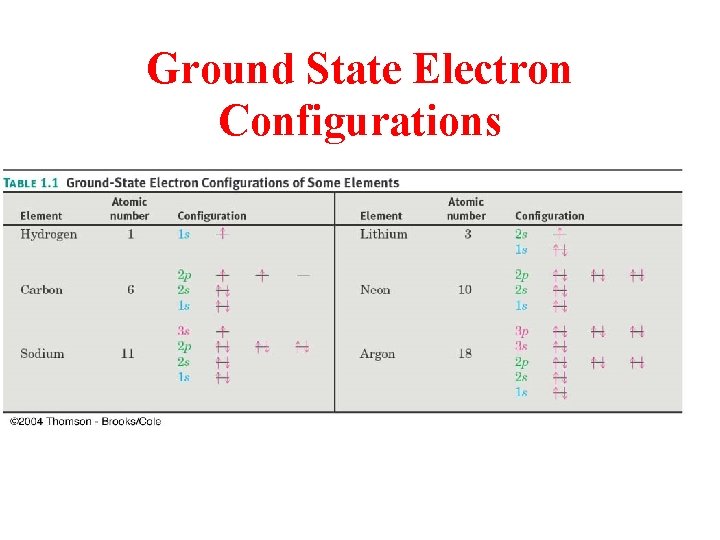
Ground State Electron Configurations
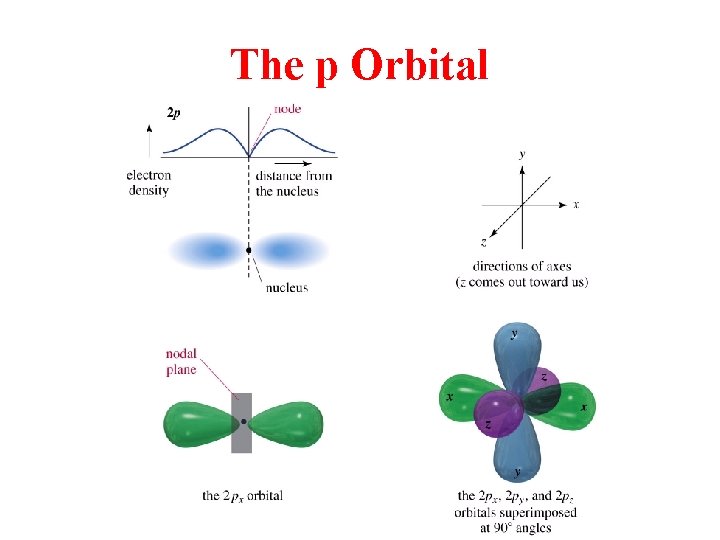
The p Orbital
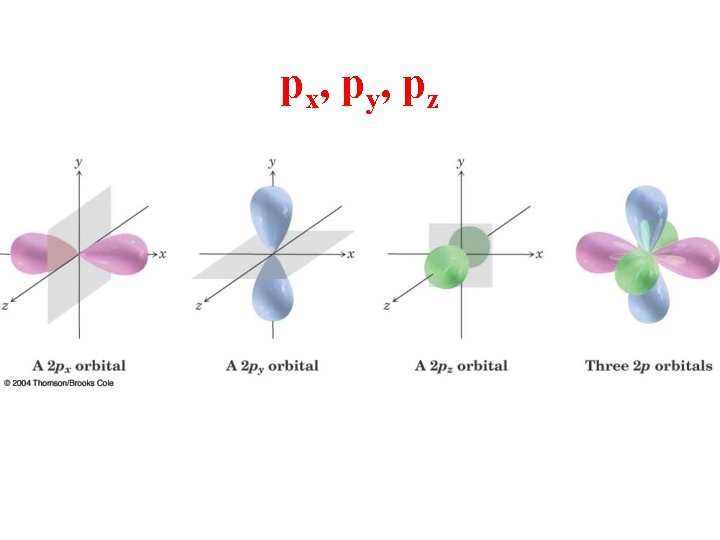
px, py, pz
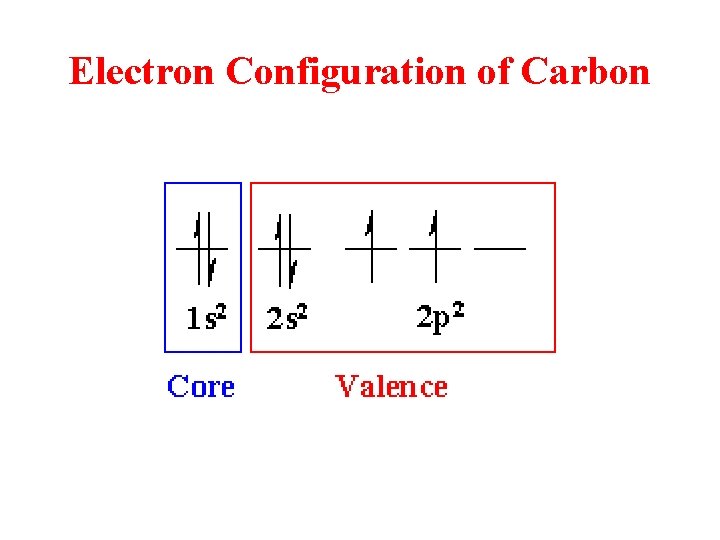
Electron Configuration of Carbon
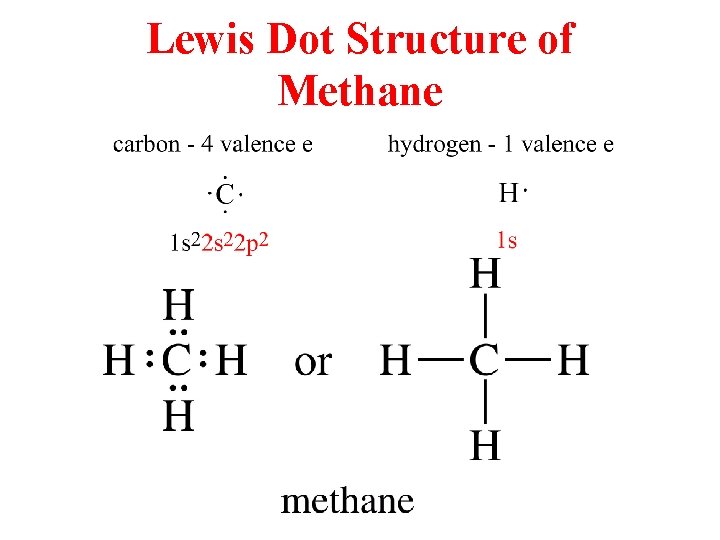
Lewis Dot Structure of Methane
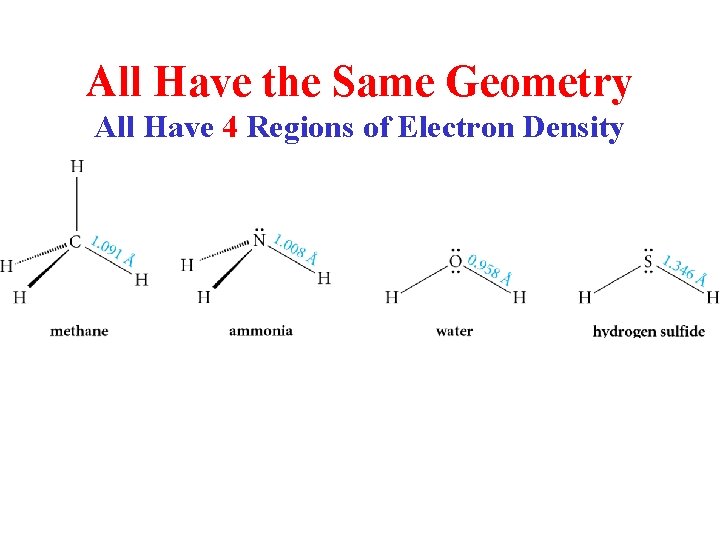
All Have the Same Geometry All Have 4 Regions of Electron Density
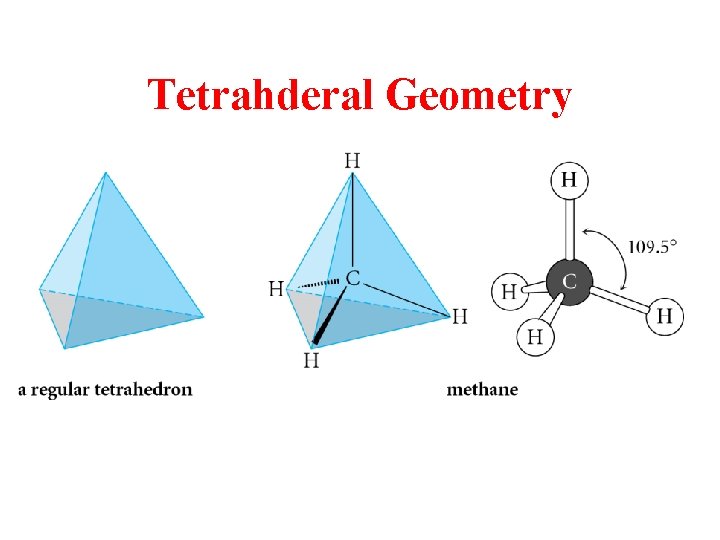
Tetrahderal Geometry
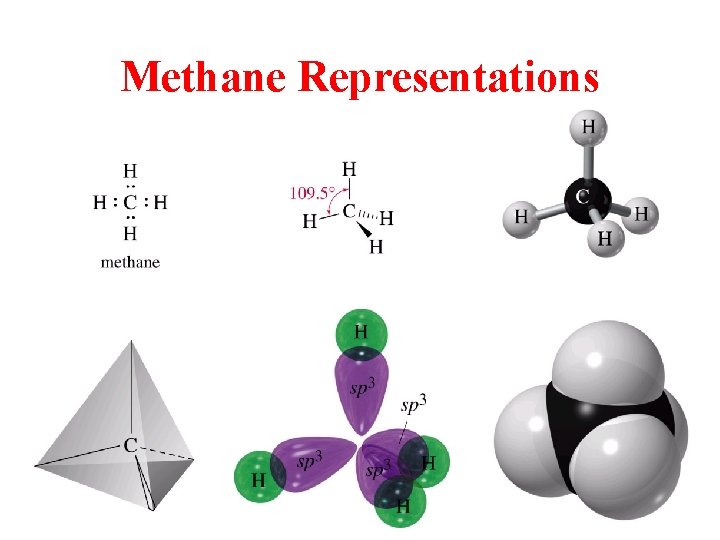
Methane Representations
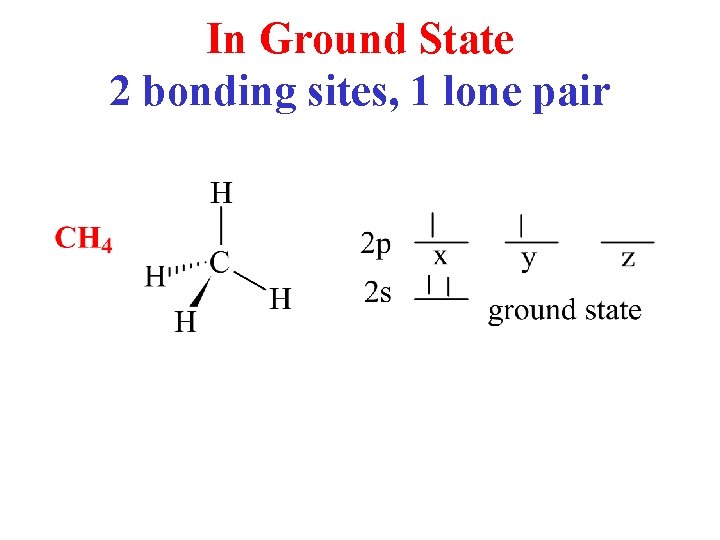
In Ground State 2 bonding sites, 1 lone pair
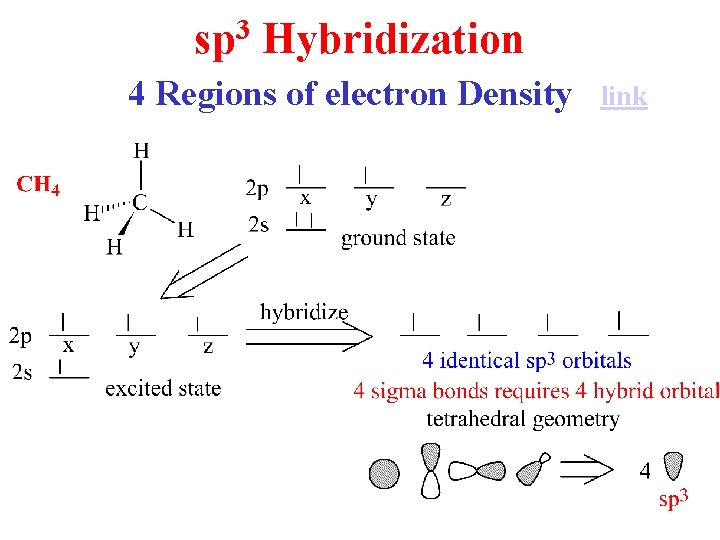
3 sp Hybridization 4 Regions of electron Density link
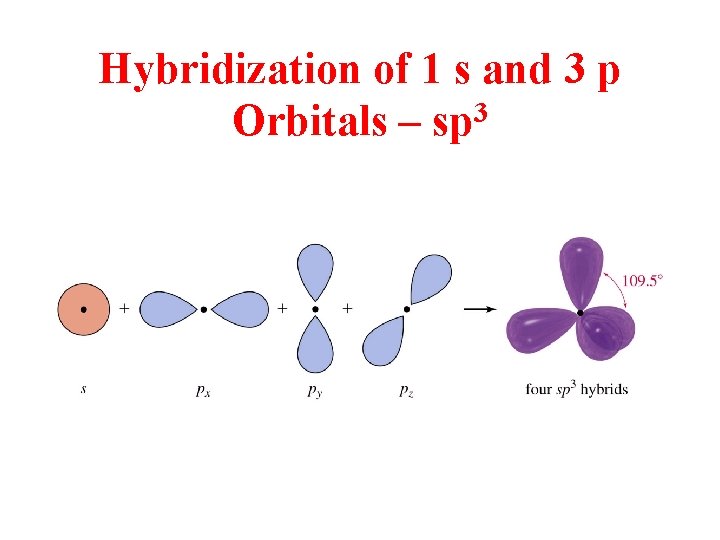
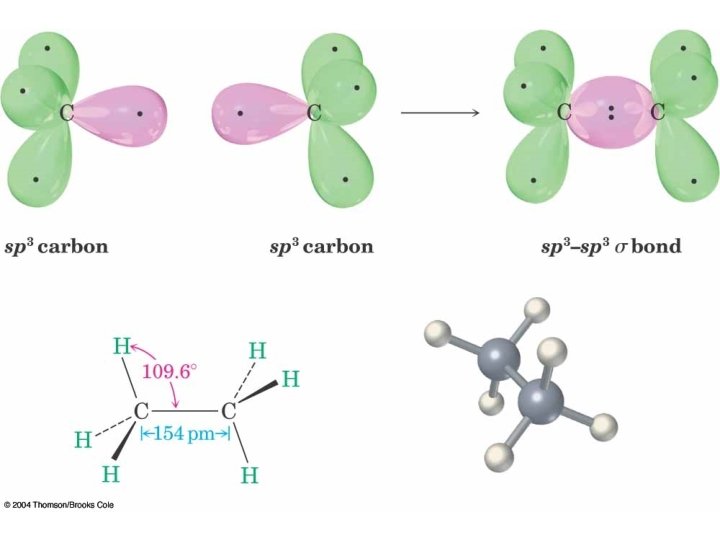
Hybridization of 1 s and 3 p Orbitals – sp 3
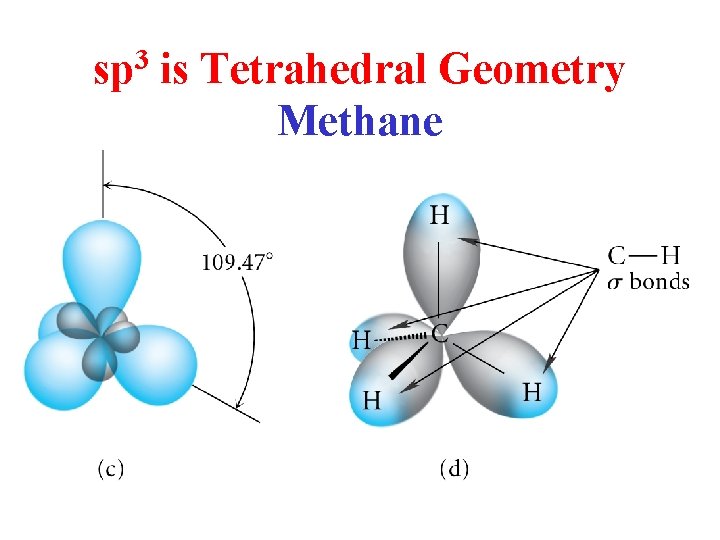
sp 3 is Tetrahedral Geometry Methane
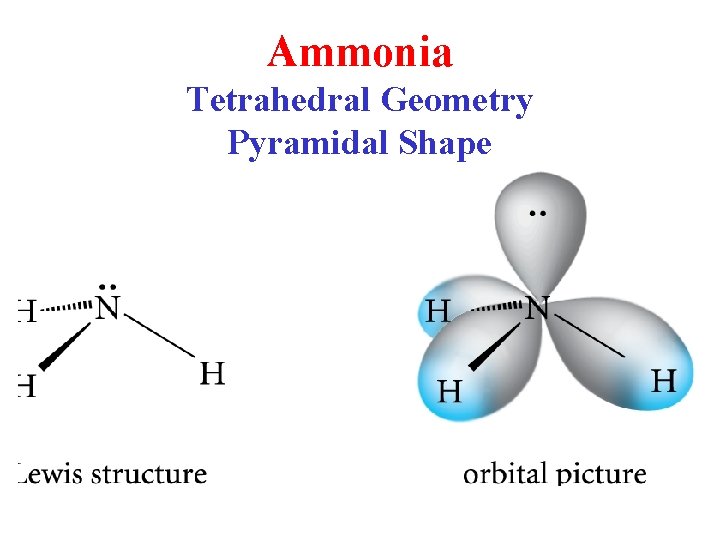
Ammonia Tetrahedral Geometry Pyramidal Shape
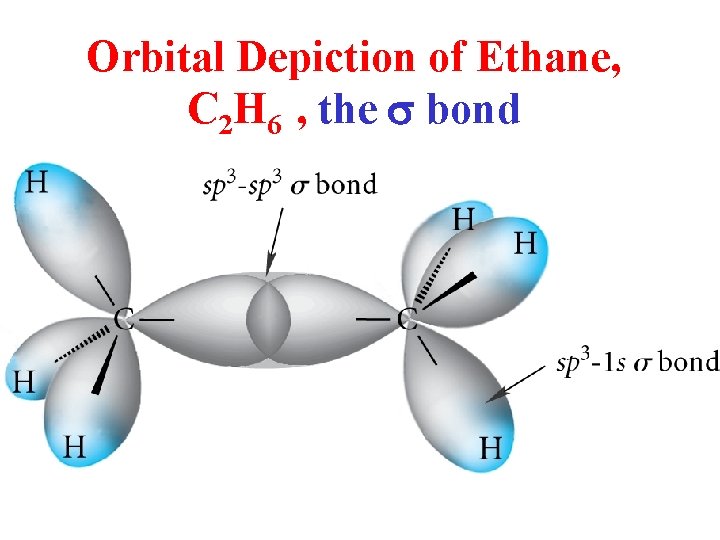
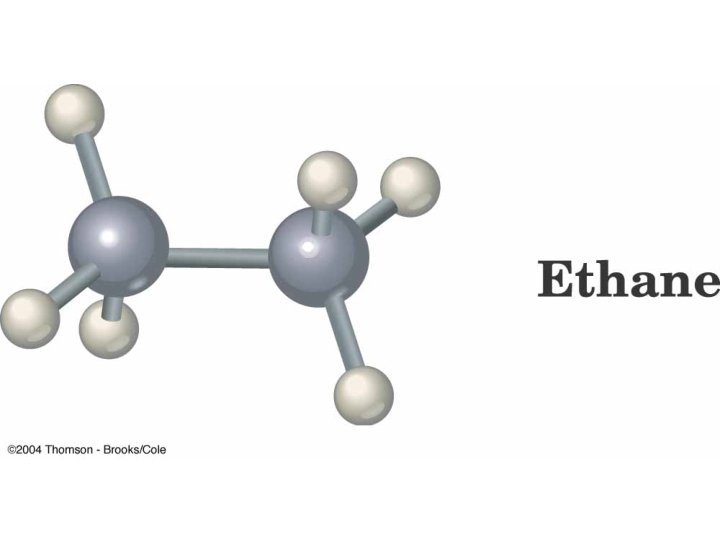
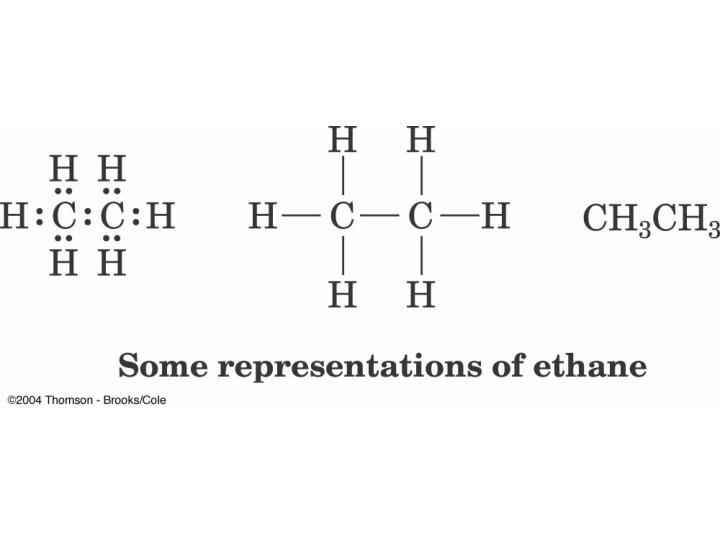
Orbital Depiction of Ethane, C 2 H 6 , the s bond
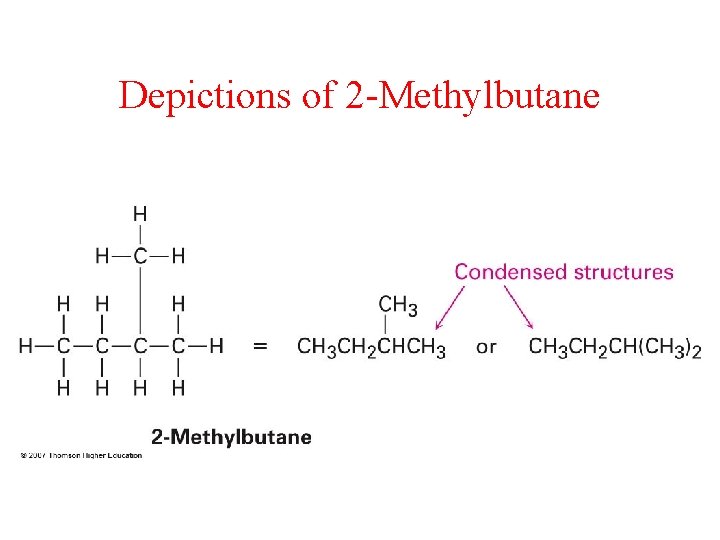
Depictions of 2 -Methylbutane
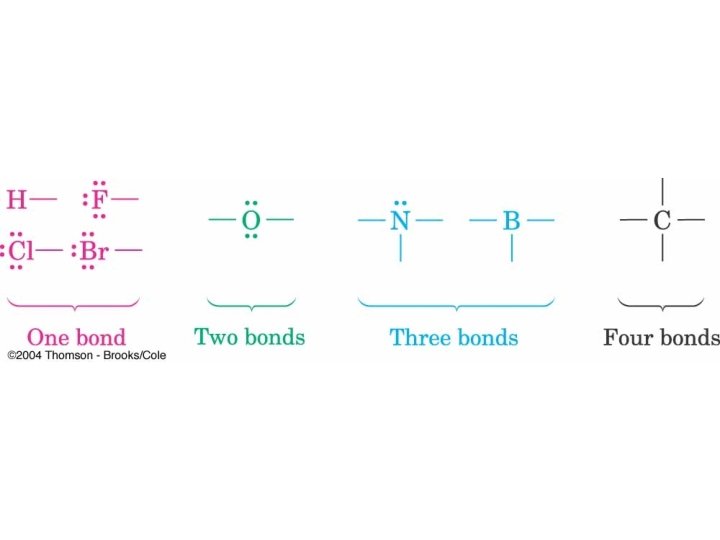
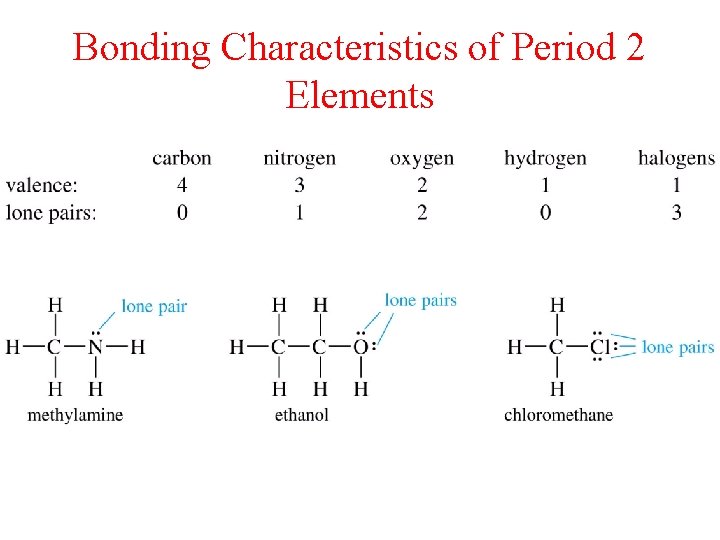
Bonding Characteristics of Period 2 Elements
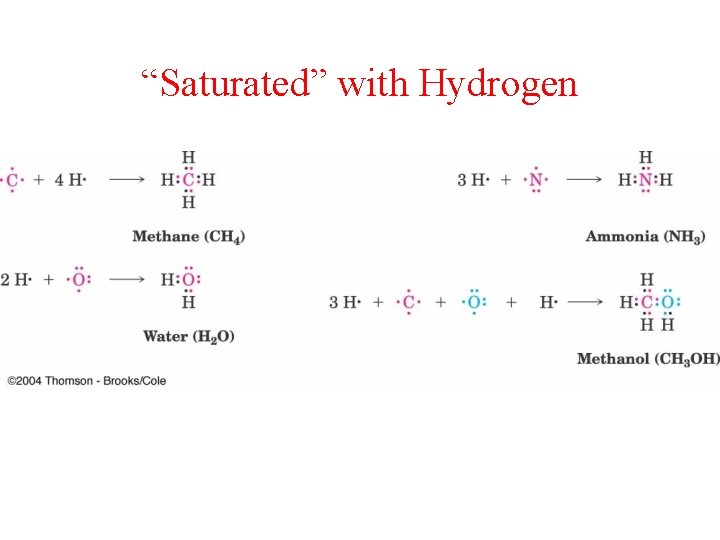
“Saturated” with Hydrogen
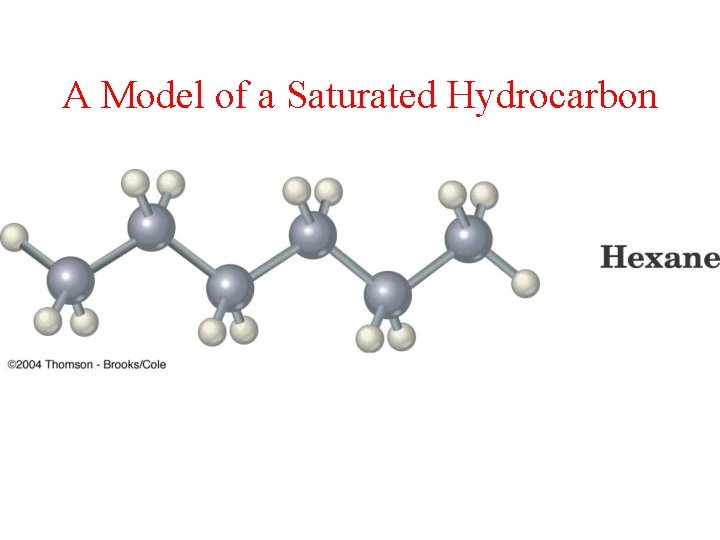
A Model of a Saturated Hydrocarbon
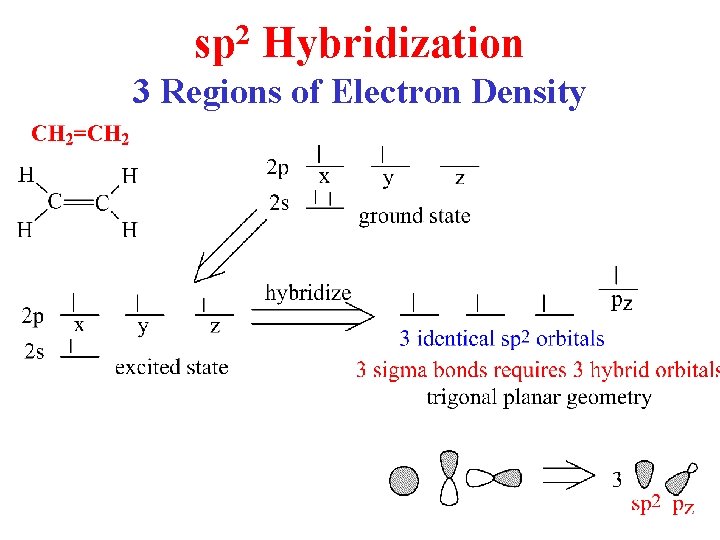
sp 2 Hybridization 3 Regions of Electron Density
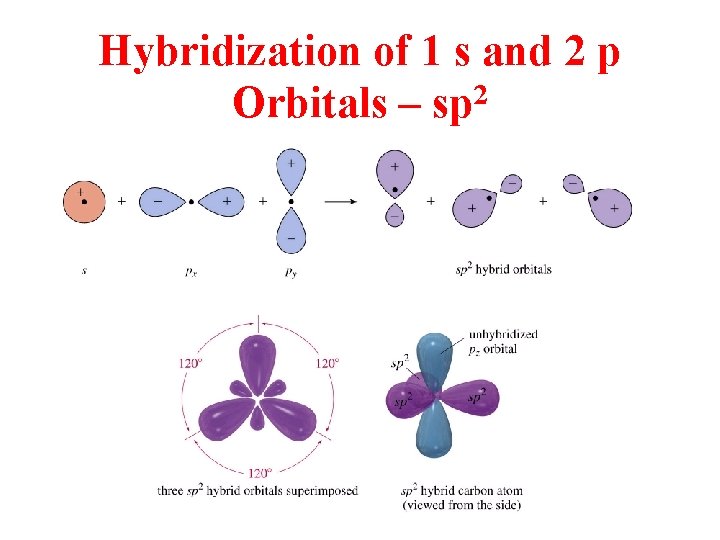
Hybridization of 1 s and 2 p Orbitals – sp 2
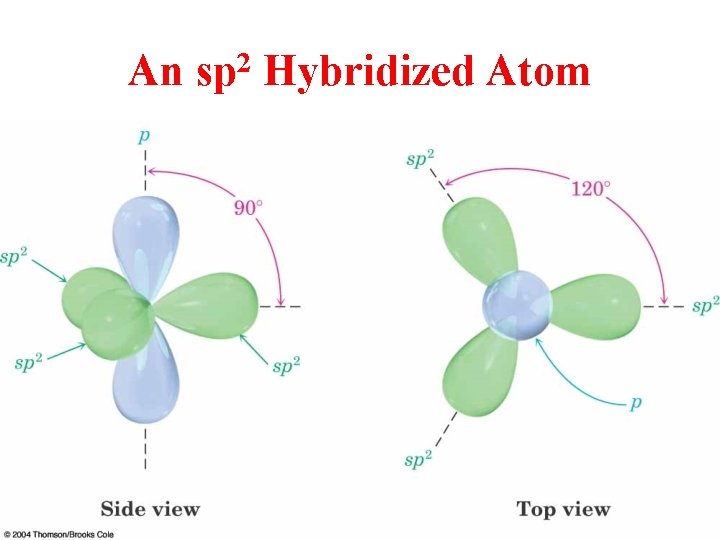
An 2 sp Hybridized Atom
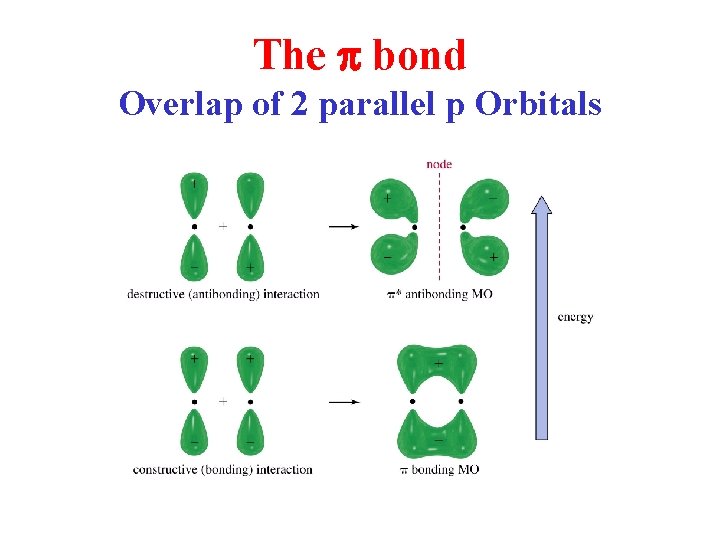
The p bond Overlap of 2 parallel p Orbitals
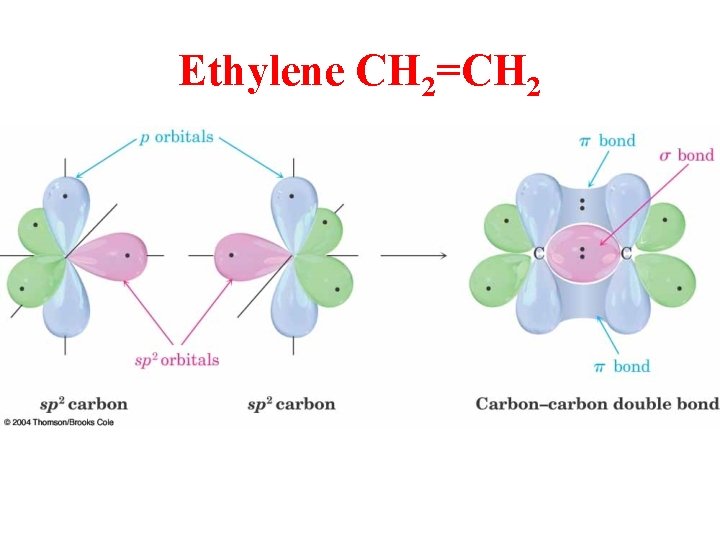
Ethylene CH 2=CH 2
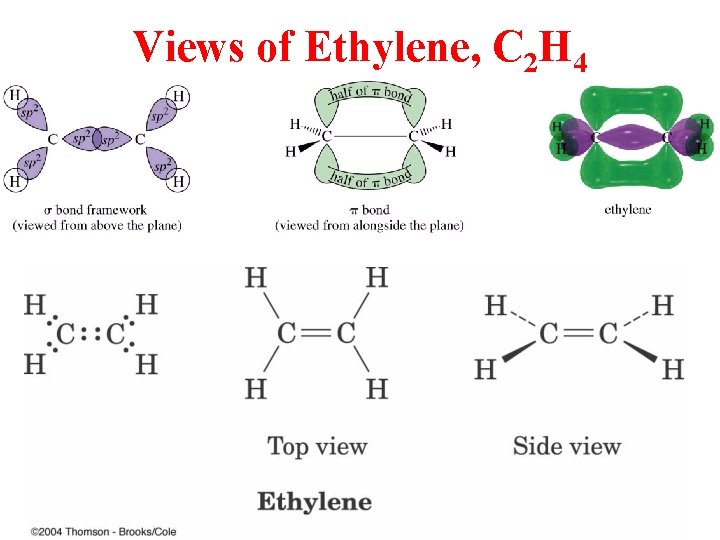
Views of Ethylene, C 2 H 4
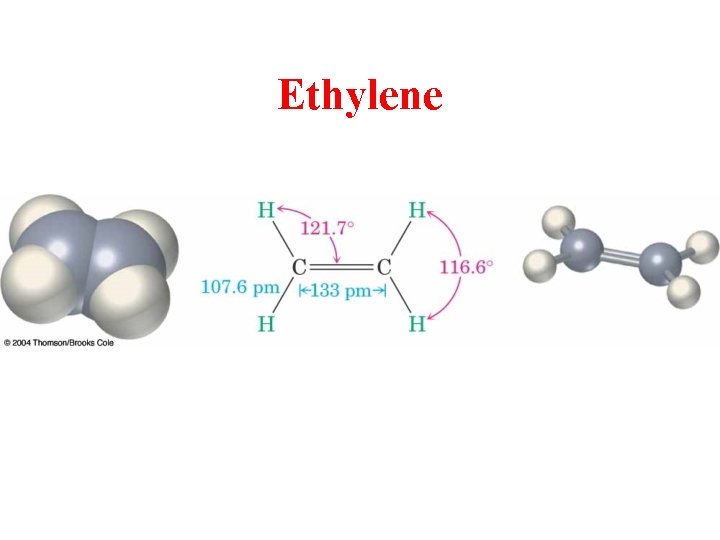
Ethylene
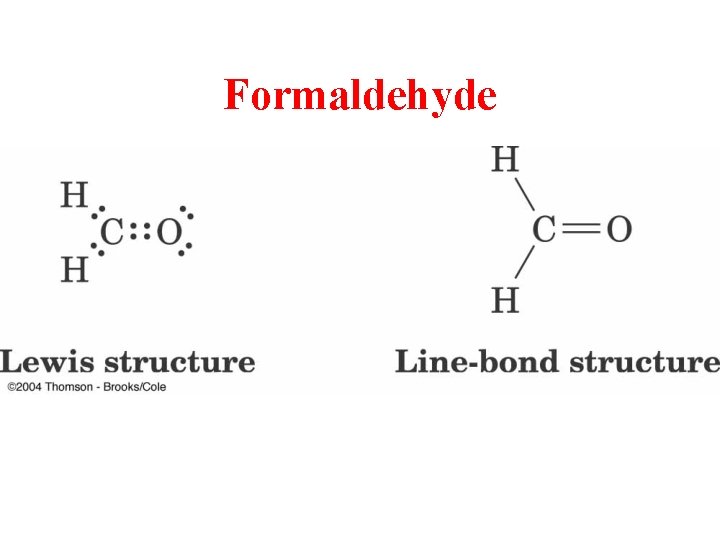
Formaldehyde
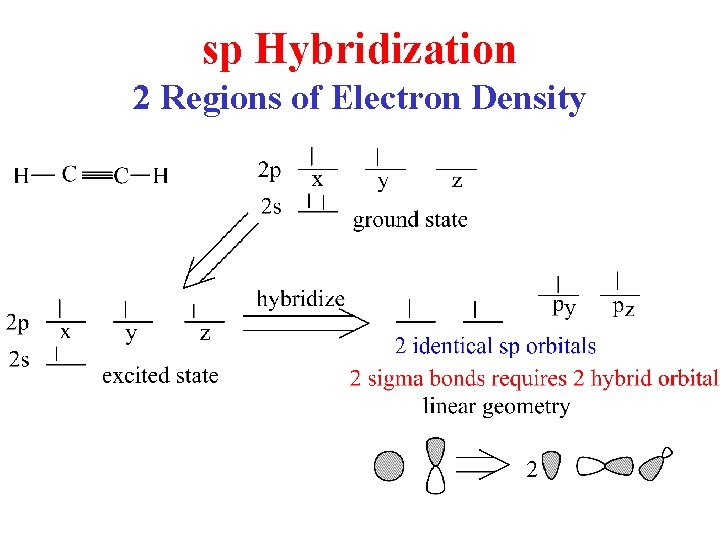
sp Hybridization 2 Regions of Electron Density
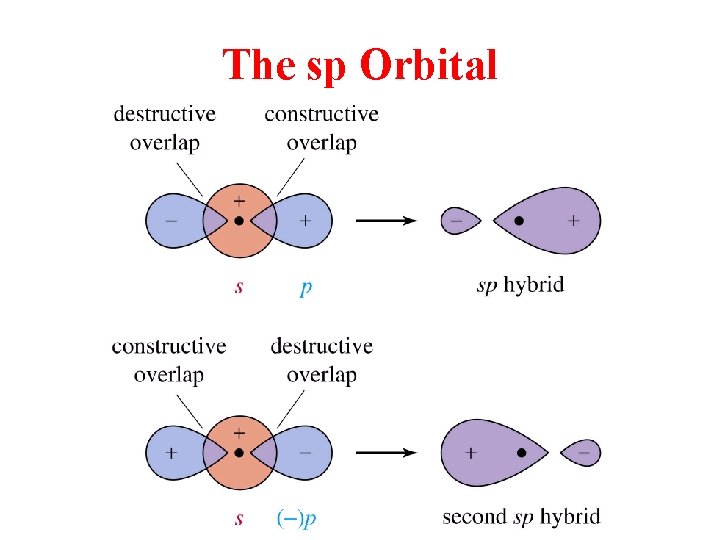
The sp Orbital
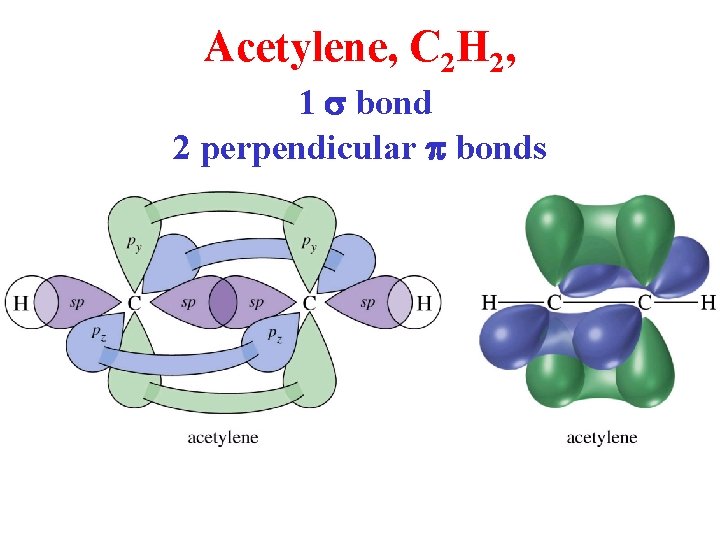
Acetylene, C 2 H 2, 1 s bond 2 perpendicular p bonds
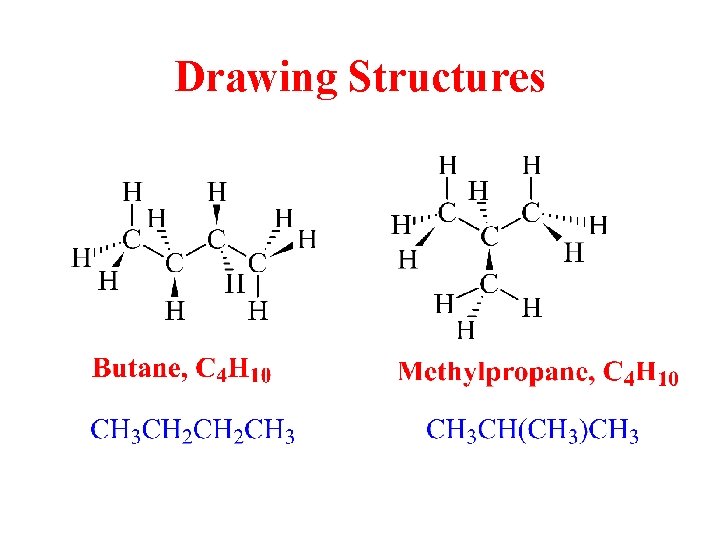
Drawing Structures
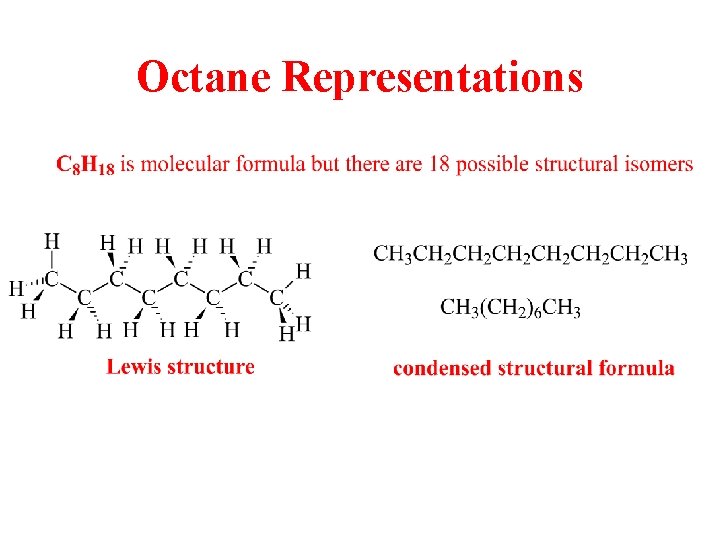
Octane Representations
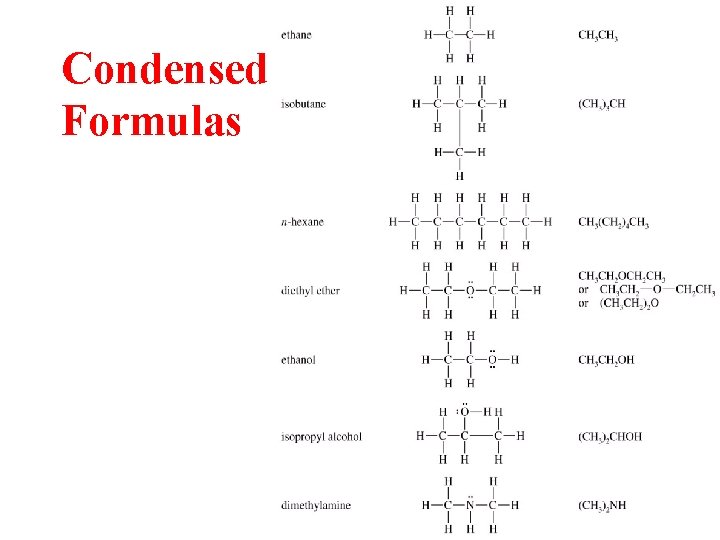
Condensed Formulas
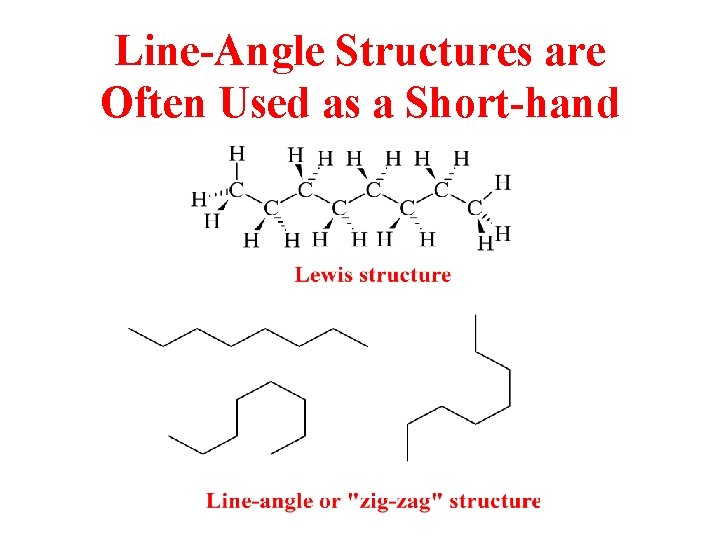
Line-Angle Structures are Often Used as a Short-hand
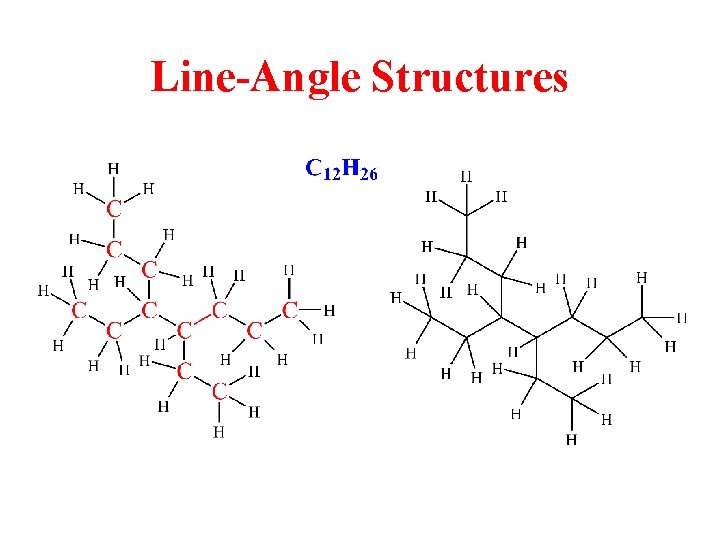
Line-Angle Structures
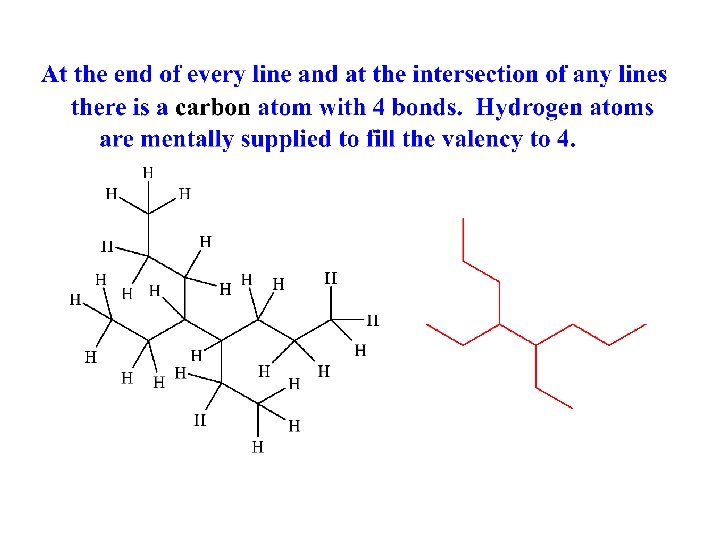
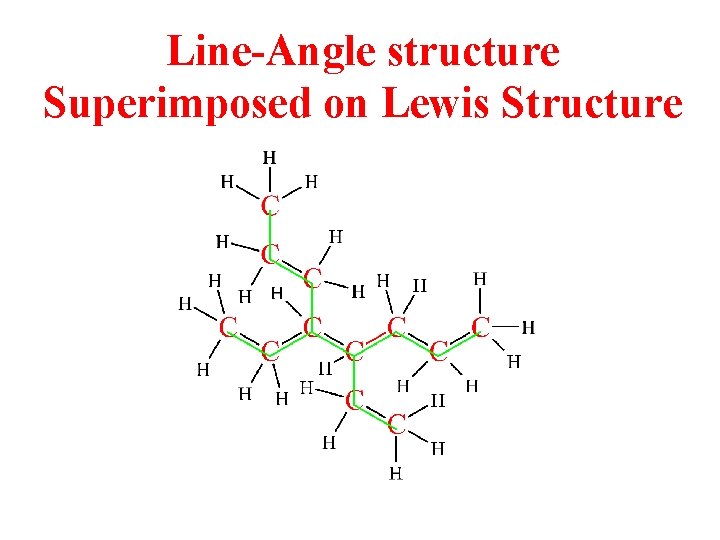
Line-Angle structure Superimposed on Lewis Structure
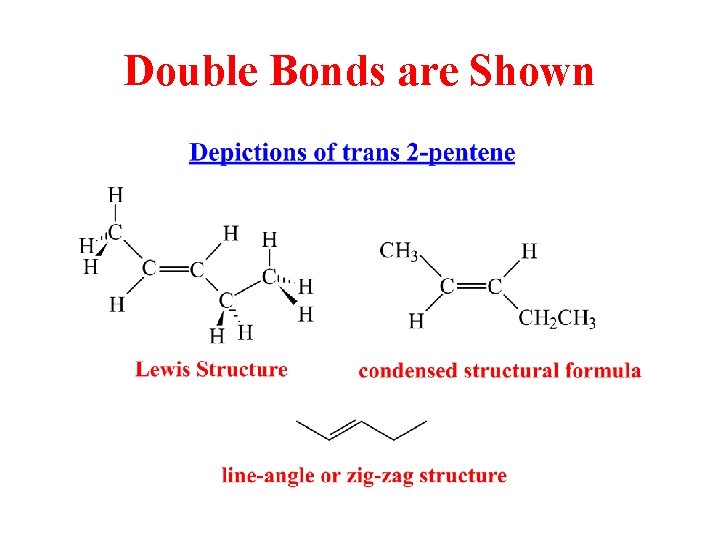
Double Bonds are Shown
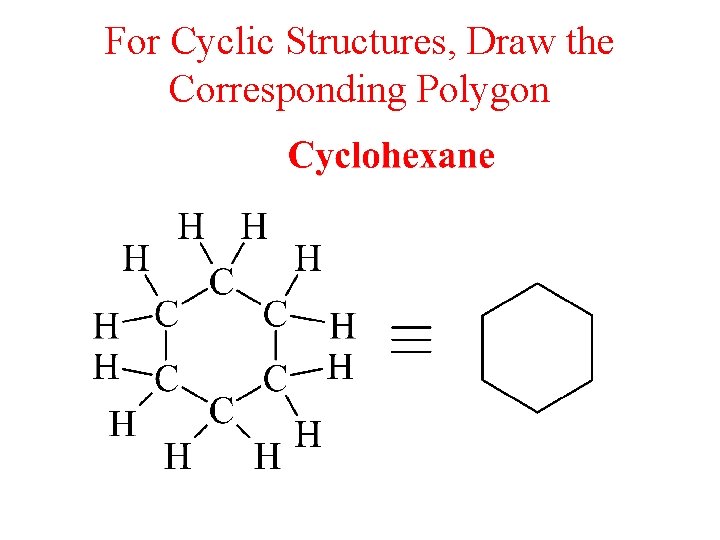
For Cyclic Structures, Draw the Corresponding Polygon
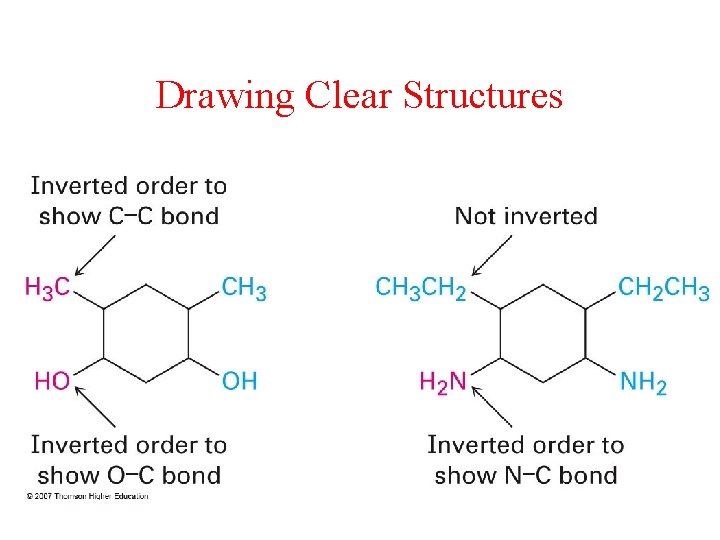
Drawing Clear Structures
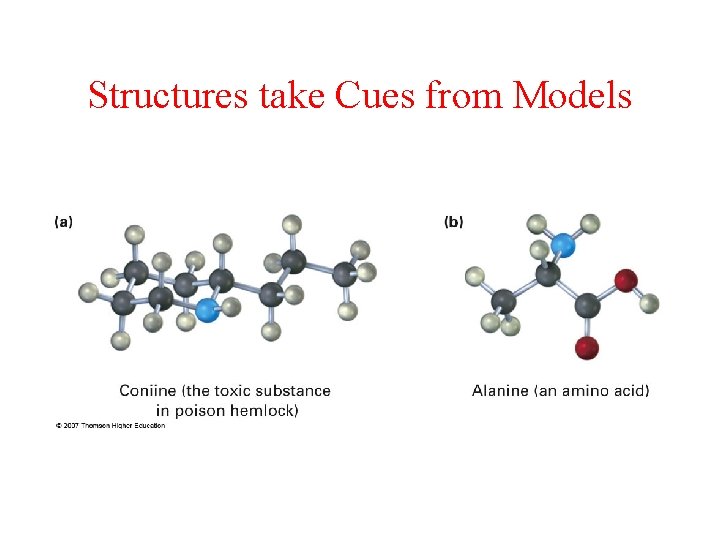
Structures take Cues from Models
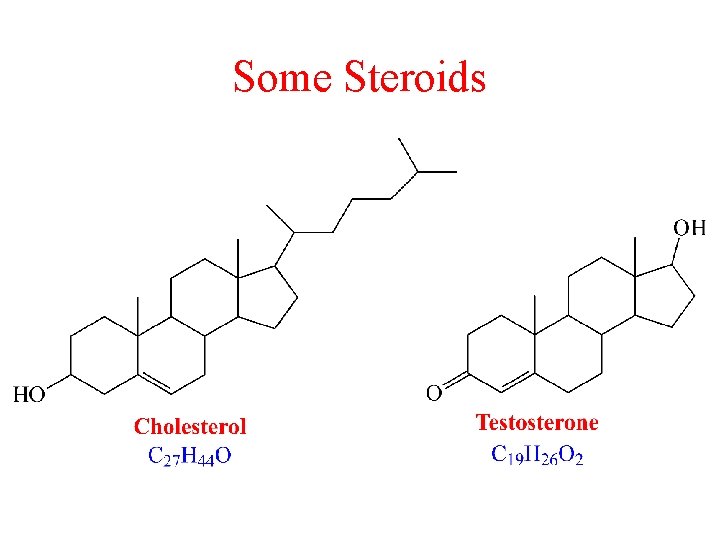
Some Steroids
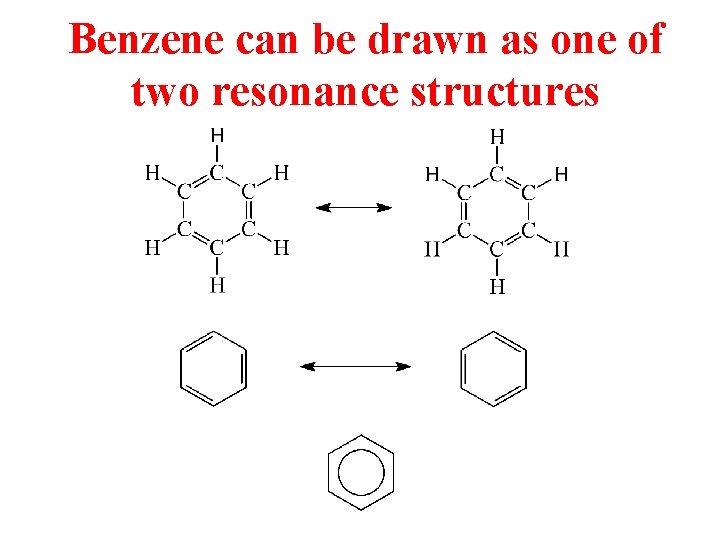
Benzene can be drawn as one of two resonance structures
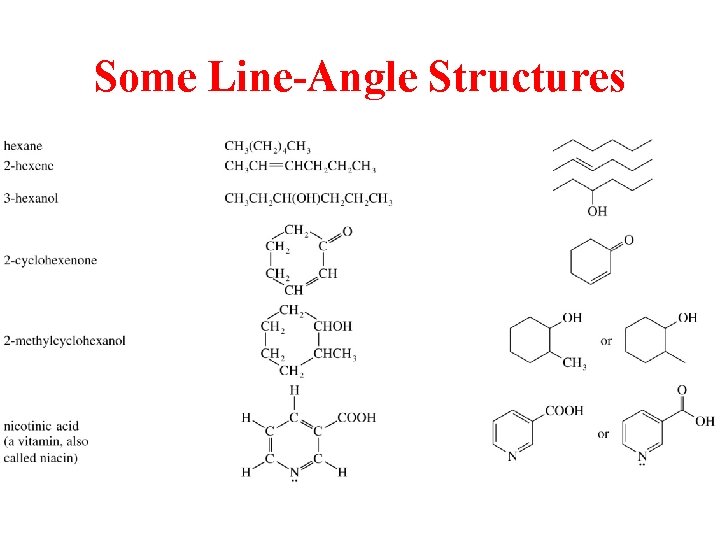
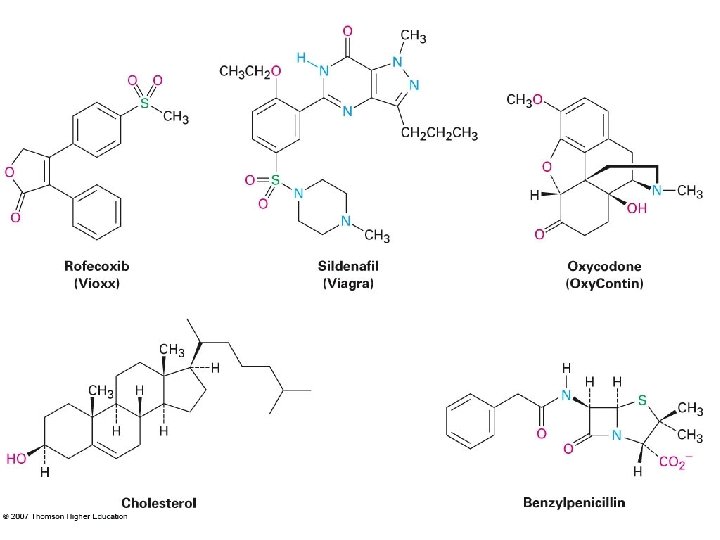
Some Line-Angle Structures
- Slides: 63