Structural isomers Naming Branched Alkanes Mr Shields Regents

-Structural isomers -Naming Branched Alkanes Mr. Shields Regents Chemistry U 16 L 02 1
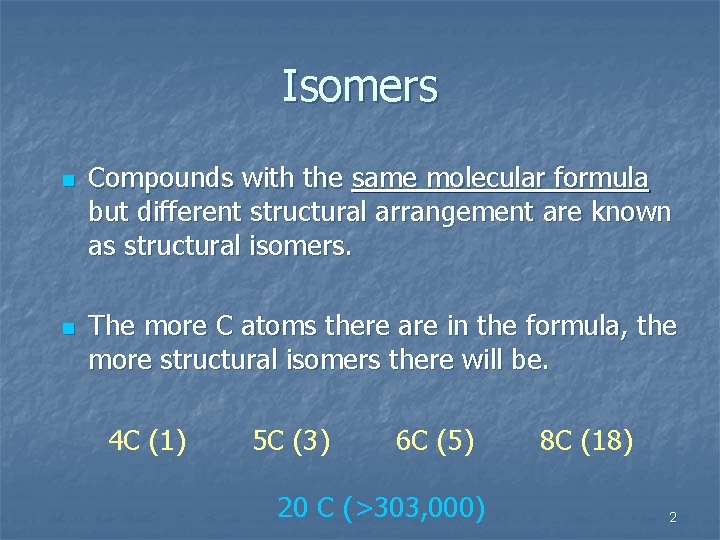
Isomers n n Compounds with the same molecular formula but different structural arrangement are known as structural isomers. The more C atoms there are in the formula, the more structural isomers there will be. 4 C (1) 5 C (3) 6 C (5) 20 C (>303, 000) 8 C (18) 2

n Structural Isomers In order to determine if 2 n To the right we have molecules are isomers of one another first determine the molecular formula’s of both n n n pentane (Top) 2 – methyl butane (bottom) What is the mol. Formula for each? Are they structural isomers? C 5 H 12 – Yes, they Are isomers 3

Isomers n Isomers have different structures and different chemical and physical properties. Formula B. P. M. P. Density Sol. In 100 ml Alcohol Butane C 4 H 10 0 C -138 C 0. 622 1813 ml 2 -methylpropane C 4 H 10 -12 C -159 C 0. 604 1320 ml NOTE! CH 3 CH 2 OH and CH 3 OCH 3 are also isomers of one another 4

Drawing simple alkanes Recall that the members of the group of alkanes Forms a homologous series and each member of This series differs from the last by 1 –CH 2 - unit When we draw the structural formulas of the 1 st Three members of this group there is only one Way each can be drawn. CH 4 CH 3 -CH 3 -CH 2 -CH 3 5

Branched-chain alkanes n n Beginning with butane, C 4 H 10, there is more than 1 way to arrange the atoms besides one carbon after another (i. e. straight chain). Can you figure out how many ways it can be drawn? CH 3 -CH 2 -CH 3 & CH 3 | CH 3 -CH-CH 3 6

Branched-chain alkanes n n n In both butane structures we have the same numbers and kind of atoms namely, C 4 H 10 The general formula for each is also Cn. H 2 n+2 so each represents the alkane “Butane” Yet there is a difference. The difference lies in what atoms are joined to what atoms CH 3 -CH 2 -CH 3 * CH CH 3 | C 4 H 10 4 10 CH 3 -CH-CH 3 * having Note that these are totally different compounds there own unique Chemical & Physical Properties! 7

Note that we have drawn for butane represents Real structural changes and not just apparent changes Resulting from the rotation of a C-C single bond. Let’s Look a pentane to see what we mean by this. Rotation of this bond Leads to this configuration Of pentane And rotation of this Bond leads to this All of these structures represent the SAME molecule! 8

Pentane isomers To find new structural isomers of the straight chain Alkanes we need to move the point of attachment of The various carbon atoms. So how many structural Isomers does pentane Have? Remember, carbon Must have 4 bonds. No more, No less! Straight chain isomer 9

Naming branched-chain alkanes n Find the longest continuous chain or backbone of C atoms. c-c-c-c-c-c c n What’s the longest chain? The base name is derived from the number of C’s in the longest chain. 10 carbons would be decane 10

Naming branched-chain alkanes n n Branches are added as a prefix and are named by counting the number of C atoms. The “branch” alkane name ends in “yl. ” c-c-c-c-c-c c Methyl CH 3— Ethyl CH 3 CH 2 – Propyl CH 3 CH 2 – Butyl CH 3 CH 2 C H 2 – 11

Naming branched-chain alkanes n n The location of the branch (or substituent group) is shown by assigning numbers to the C’s in the backbone. Number from the end that gives the lowest number for the first branch. n Substituent groups (branches) are listed alphabetically 1 2 3 4 5 6 7 8 9 10 c-c-c-c-c-c c c 7 -ethyl-3 -methyldecane c n. There may be more than 1 of the same type of branch. n Use di, tri, tetra etc. for 2, 3, and 4 n Number the locations and separate the nos. by a comma & 12 separate the last no. from the name by a dash

Name this compound CH 3 | H 3 C-C-CH 2 -CH 3 | CH 3 Longest continuous chain has 4 carbon atoms – butane. 2 Branches each have 1 carbon – dimethyl. Branches are at C-2. WHY? ? 2, 2 -Dimethylbutane or C 6 H 14 or CH 3 C(CH 3)2 CH 3 13

H H–C–H H H H–C–C–C–H H H Example: H-C-H | H Longest continuous chain has 3 carbon atoms – propane. 2 Branches each have 1 carbon – dimethyl. And … both Branches have to be at C-2. WHY? ? So …. Only specify the branch number if necessary. Ex: this is Dimethylpropane & not 2, 2 -dimethylpropane 14

Name this compound: H H–C–H H H– C–C–H H H H–C–H H H Longest continuous chain has 6 carbon atoms: hexane Branch is 1 carbon long: methyl Branch is located at C-3 3 -methylhexane or C 7 H 16 or CH 3 CH 2 CH(CH 3)CH 2 CH 3 15

More Naming Problems Methylbutane 2, 3, 7 -Trimethyloctane Dimethylpropane 3 -ethylpentane CH 3 CH(CH 3)CH 2 CH 2 CH(CH 3)CH 3 16

More Naming Problems C C C | | | C-C-C-C | C What is the name Of this compound ? 4 -ethyl-2, 5 -dimethyloctane WOW ! 17

Problems: n Draw the structural formula for the following: n n methylpropane 3 – ethyl – 4 methylnonane dimethylpropane 2, 3, 4 – trimethyldecane 3 -ethyl-3 -methylpentane What name does this compound have? 18

Name this compound 9 C-C-C | 1 C-C-C-C-C | | | CC C | C 6, 6 -diethyl-3, 5 dimethylnonane 19
- Slides: 19