Structural Heart Live Cases Supported by Medtronic Inc

Structural Heart Live Cases Supported by: • Medtronic Inc. • Bard Inc. • Terumo Medical Corp.

Faculty Involved and Disclosures Samin K. Sharma, MD, FACC, MSCAI Speaker’s Bureau – BSC, Abbott Vascular Inc, CSI Annapoorna S. Kini, MD, FACC Nothing to disclose Cardiac Anesthesiologist Muoi Trinh MD Gilbert Tang, MD, MSc, MBA, FACC Physician Proctor and consultant for Medtronic Stamatios Lerakis, MD, Ph. D, FACC, FAHA (Imaging) Nothing to disclose Pedro Moreno, MD, Ph. D [Moderator] Nothing to disclose
Structural Heart Live Case# 38: OL, 60 y/o M Presentation: Progressive fatigue and DOE NYHA Class III for 2 mths PMH: COPD, ESRD on HD, HFr. EF (25%), CAD, left arm AV fistula, Active tobacco abuse disorder, pleural effusions s/p thoracentesis (transudative), AI, Bioprosthetic AVR (23 mm Magna 3000) in 2017 Medications: ASA, Carvedilol, Hydralazine, ISMN, Renvela, Amlodpine, ASA Labs: Hgb 8. 8, PLT 157 K, K 3. 4, Cr 7. 7, INR 1. 5 EKG (11/1/20): NSR with 1 st degree AV block PR 234 msec, QTc 509 msec TTE (10/14/20): Severe bio-prosthetic AS (PG/MG/AVA/PV: 82/51/0. 6/4. 5), trace MR, trace TR and LVEF 20% Cath (10/8/20): m. RCA 50 -60%, p. LAD 60 -70%, OM 2 70 -80%; managed medically
Structural Heart Live Case #38: OL, 60 y/o M TEE (10/16/2020): LVEF-20% • Severely thickened leaflets with restricted opening • No evidence for paravalvular AI, mild central AI, mild MR, mild TR • Peak velocity = 3. 8 m/s; effective orifice area (EOA) by Doppler = 0. 9 cm^2 • No evidence for prosthesis-patient mismatch (PPM)

Trans-esophageal Echocardiogram 3 D TEE
Structural Heart Live Case: OL, 60 y/o M STS risk of mortality for AVR: 5. 88% Heart team approach: Patient evaluated by Heart Team and due to multiple co-morbidities, the patient was determined to be at high risk for 2 nd time reoperation for surgical AVR Plan: Patient determined to be a suitable candidate for Vi. V TAVR
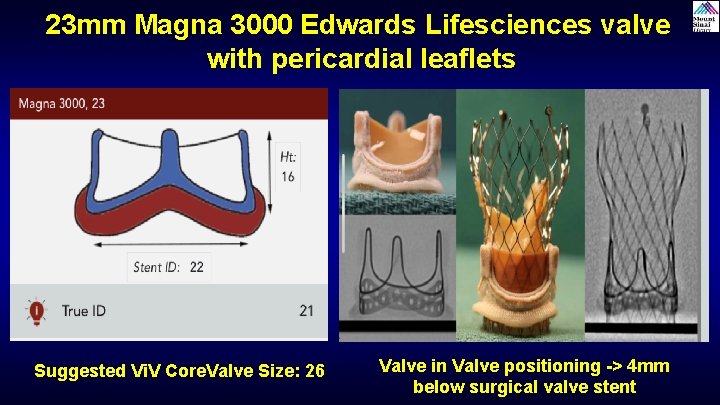
23 mm Magna 3000 Edwards Lifesciences valve with pericardial leaflets Suggested Vi. V Core. Valve Size: 26 Valve in Valve positioning -> 4 mm below surgical valve stent
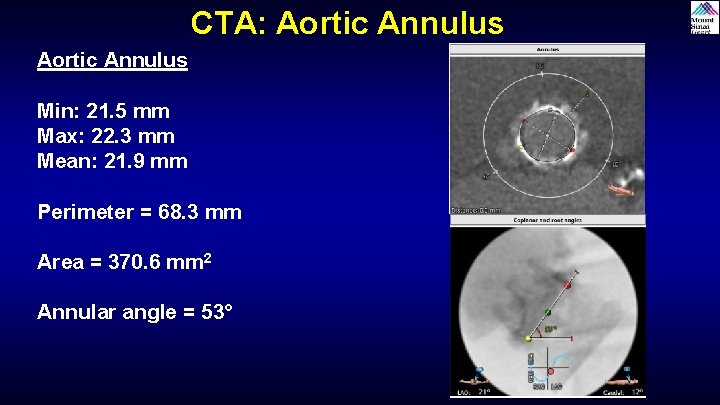
CTA: Aortic Annulus Min: 21. 5 mm Max: 22. 3 mm Mean: 21. 9 mm Perimeter = 68. 3 mm Area = 370. 6 mm 2 Annular angle = 53°
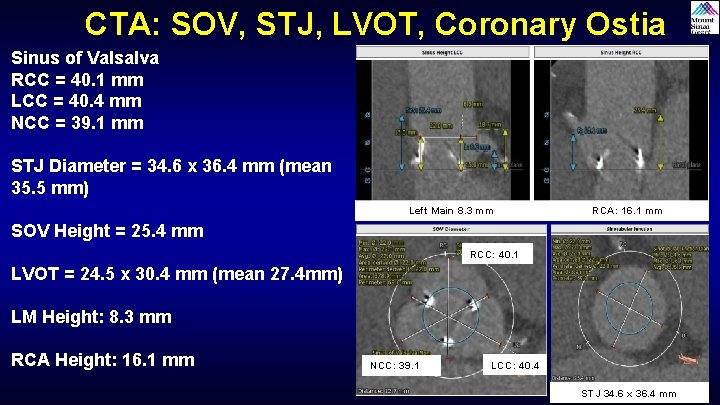
CTA: SOV, STJ, LVOT, Coronary Ostia Sinus of Valsalva RCC = 40. 1 mm LCC = 40. 4 mm NCC = 39. 1 mm STJ Diameter = 34. 6 x 36. 4 mm (mean 35. 5 mm) Left Main 8. 3 mm RCA: 16. 1 mm SOV Height = 25. 4 mm RCC: 40. 1 LVOT = 24. 5 x 30. 4 mm (mean 27. 4 mm) LM Height: 8. 3 mm RCA Height: 16. 1 mm NCC: 39. 1 LCC: 40. 4 STJ 34. 6 x 36. 4 mm
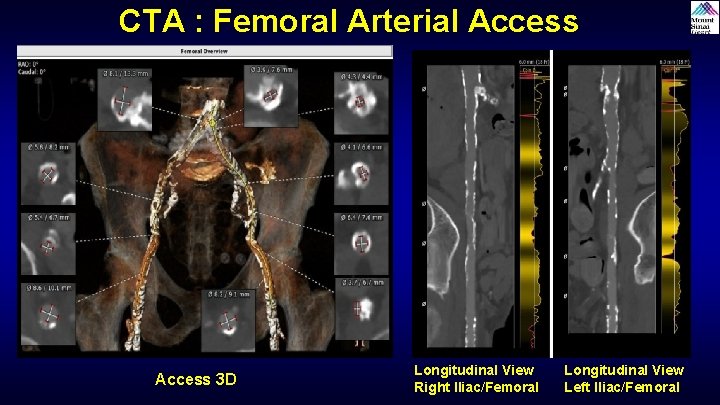
CTA : Femoral Arterial Access 3 D Longitudinal View Right Iliac/Femoral Longitudinal View Left Iliac/Femoral
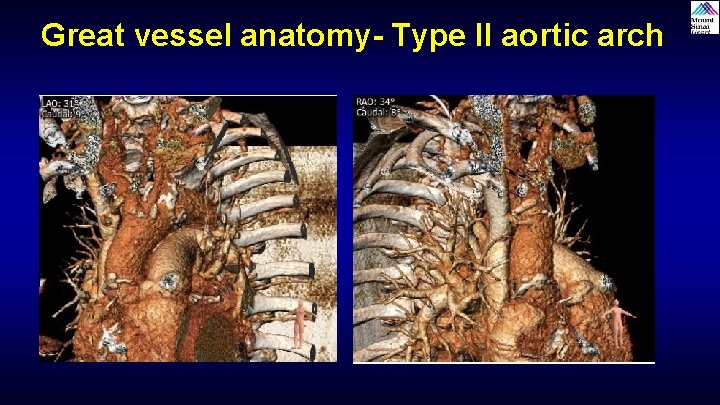
Great vessel anatomy- Type II aortic arch
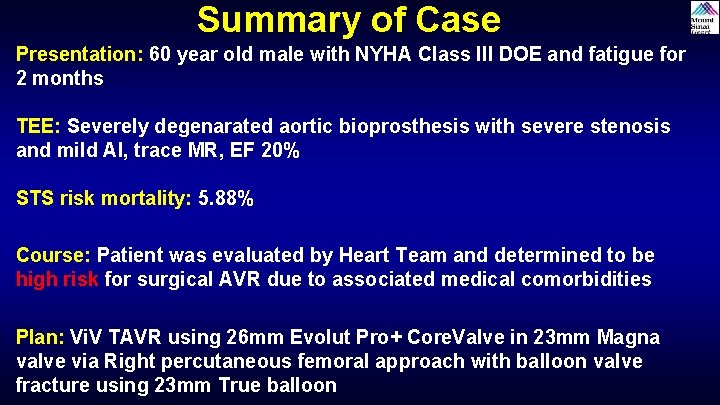
Summary of Case Presentation: 60 year old male with NYHA Class III DOE and fatigue for 2 months TEE: Severely degenarated aortic bioprosthesis with severe stenosis and mild AI, trace MR, EF 20% STS risk mortality: 5. 88% Course: Patient was evaluated by Heart Team and determined to be high risk for surgical AVR due to associated medical comorbidities Plan: Vi. V TAVR using 26 mm Evolut Pro+ Core. Valve in 23 mm Magna valve via Right percutaneous femoral approach with balloon valve fracture using 23 mm True balloon
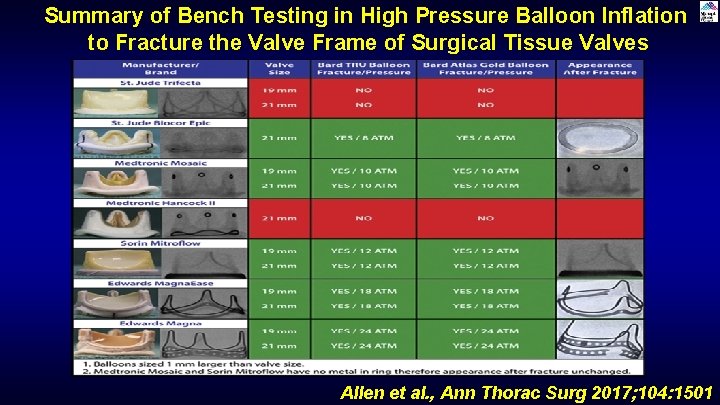
Summary of Bench Testing in High Pressure Balloon Inflation to Fracture the Valve Frame of Surgical Tissue Valves Allen et al. , Ann Thorac Surg 2017; 104: 1501
Latest Issues in Transcatheter Structural Heart Interventions • Update in Vi. V TAVR vs redo SAVR in Bio-prosthetic aortic valve degeneration
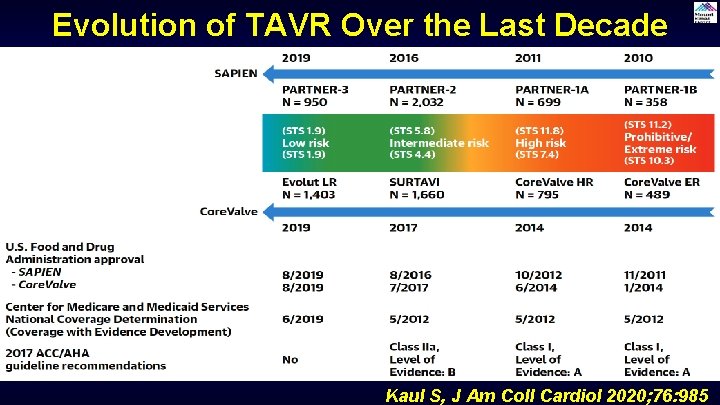
Evolution of TAVR Over the Last Decade Kaul S, J Am Coll Cardiol 2020; 76: 985
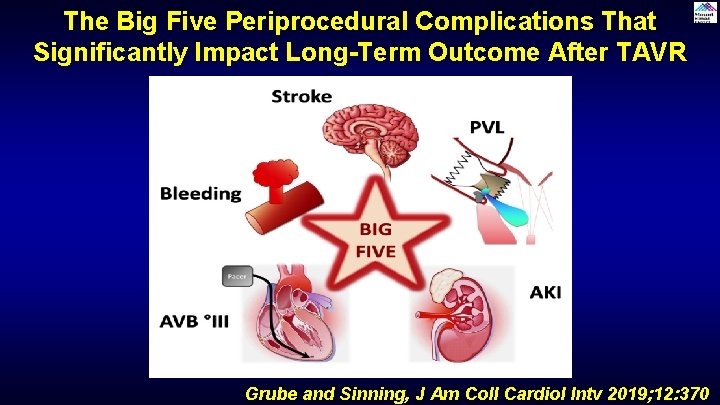
The Big Five Periprocedural Complications That Significantly Impact Long-Term Outcome After TAVR Grube and Sinning, J Am Coll Cardiol Intv 2019; 12: 370
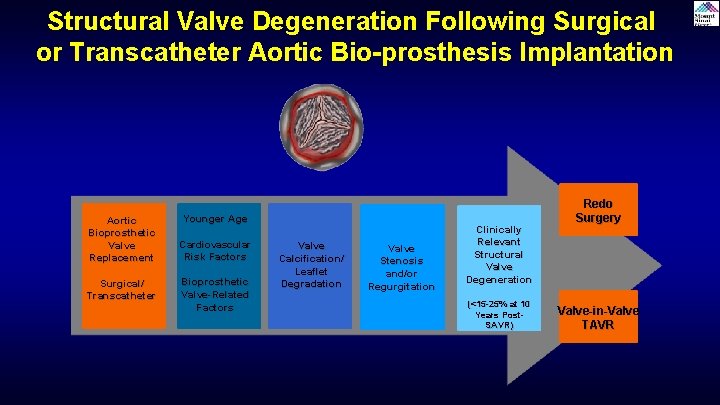
Structural Valve Degeneration Following Surgical or Transcatheter Aortic Bio-prosthesis Implantation Aortic Bioprosthetic Valve Replacement Surgical/ Transcatheter Younger Age Cardiovascular Risk Factors Bioprosthetic Valve-Related Factors Valve Calcification/ Leaflet Degradation Valve Stenosis and/or Regurgitation Clinically Relevant Structural Valve Degeneration (<15 -25% at 10 Years Post. SAVR) Redo Surgery Valve-in-Valve TAVR
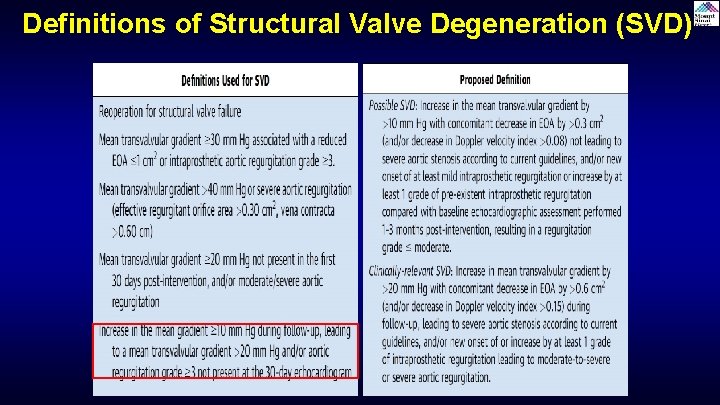
Definitions of Structural Valve Degeneration (SVD)
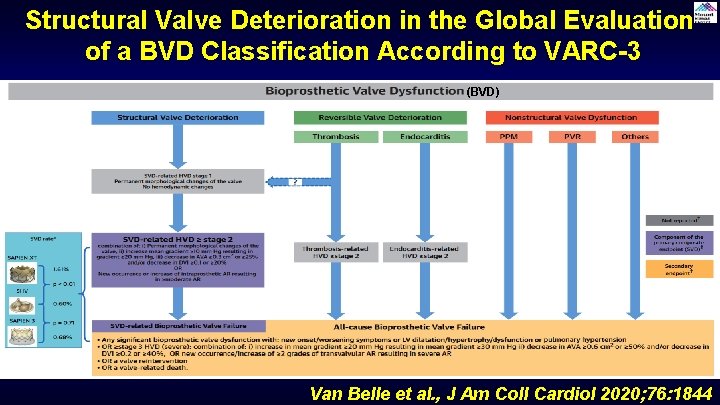
Structural Valve Deterioration in the Global Evaluation of a BVD Classification According to VARC-3 (BVD) Van Belle et al. , J Am Coll Cardiol 2020; 76: 1844
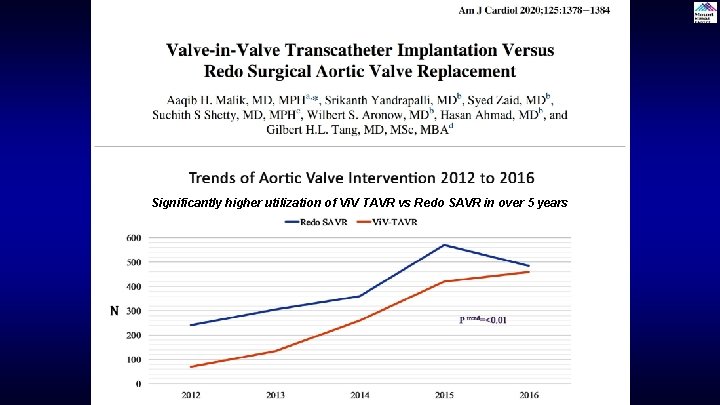
Significantly higher utilization of Vi. V TAVR vs Redo SAVR in over 5 years
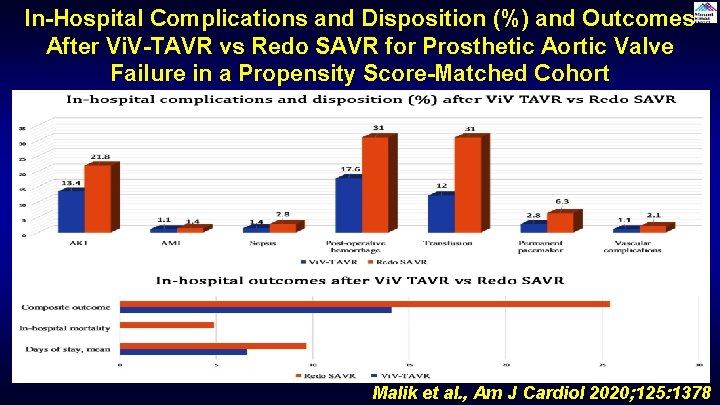
In-Hospital Complications and Disposition (%) and Outcomes After Vi. V-TAVR vs Redo SAVR for Prosthetic Aortic Valve Failure in a Propensity Score-Matched Cohort Malik et al. , Am J Cardiol 2020; 125: 1378
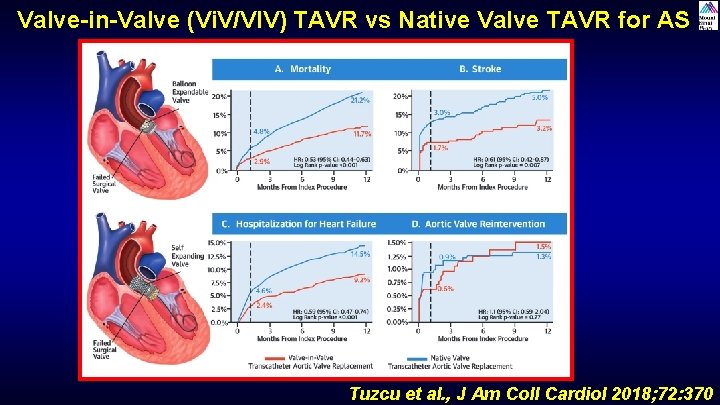
Valve-in-Valve (Vi. V/VIV) TAVR vs Native Valve TAVR for AS Tuzcu et al. , J Am Coll Cardiol 2018; 72: 370
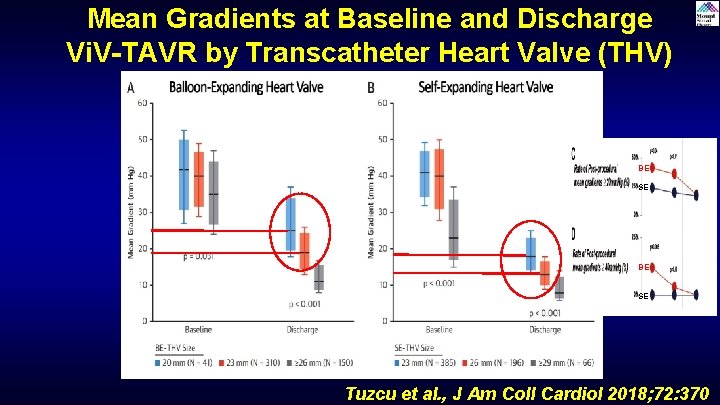
Mean Gradients at Baseline and Discharge Vi. V-TAVR by Transcatheter Heart Valve (THV) Type BE SE Tuzcu et al. , J Am Coll Cardiol 2018; 72: 370

Administrative database of Ontario, Canada About the short & 5 Yrs outcomes of failed bio-AV: Vi. V TAVR (n= 214) or Redo SAVR (n= 344) Of these 131 in each gp were propensity matched
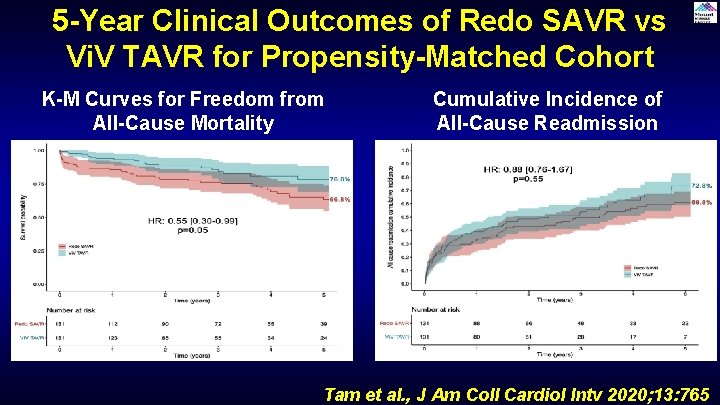
5 -Year Clinical Outcomes of Redo SAVR vs Vi. V TAVR for Propensity-Matched Cohort K-M Curves for Freedom from All-Cause Mortality Cumulative Incidence of All-Cause Readmission Tam et al. , J Am Coll Cardiol Intv 2020; 13: 765
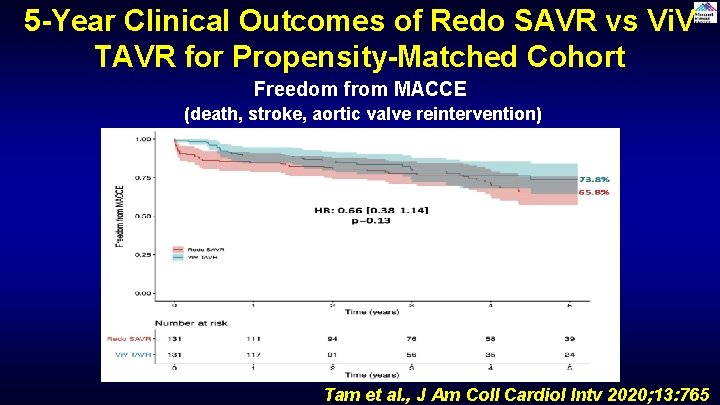
5 -Year Clinical Outcomes of Redo SAVR vs Vi. V TAVR for Propensity-Matched Cohort Freedom from MACCE (death, stroke, aortic valve reintervention) Tam et al. , J Am Coll Cardiol Intv 2020; 13: 765
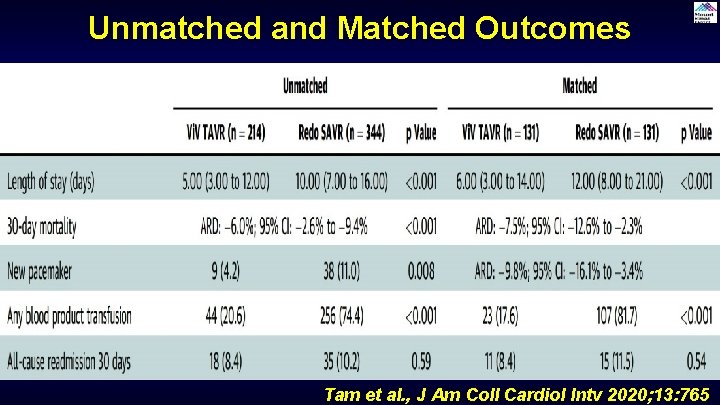
Unmatched and Matched Outcomes Tam et al. , J Am Coll Cardiol Intv 2020; 13: 765
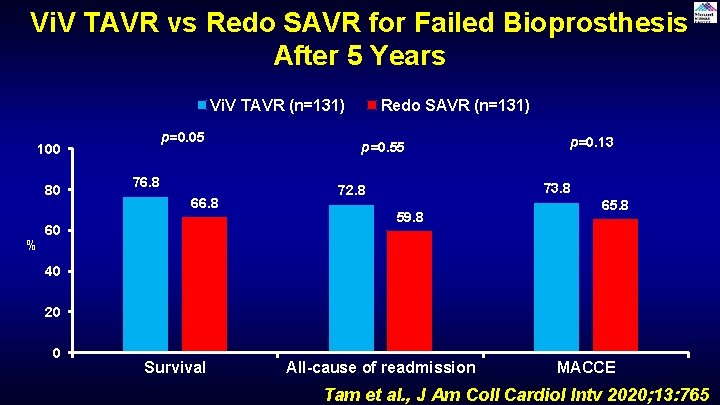
Vi. V TAVR vs Redo SAVR for Failed Bioprosthesis After 5 Years Vi. V TAVR (n=131) p=0. 05 100 80 % 76. 8 60 Redo SAVR (n=131) p=0. 55 p=0. 13 73. 8 72. 8 59. 8 65. 8 40 20 0 Survival All-cause of readmission MACCE Tam et al. , J Am Coll Cardiol Intv 2020; 13: 765


French Administrative Govt Mandated Database: 2010 -2019 N=4327 (717 matched pairs underwent Vi. V TAVR vs redo SAVR) Followed for approx 4 years (760+ 795 days; similar in both groups)
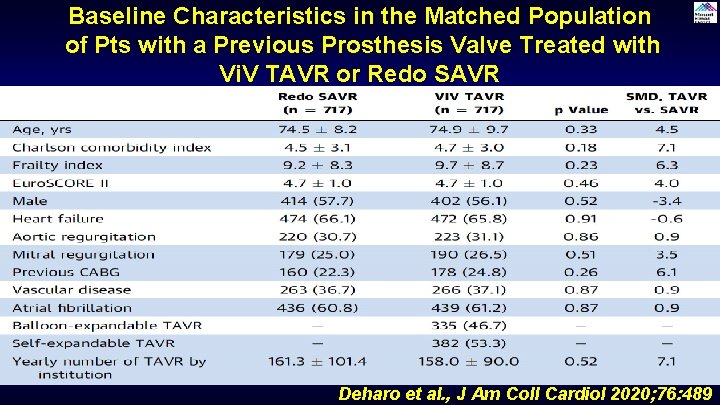
Baseline Characteristics in the Matched Population of Pts with a Previous Prosthesis Valve Treated with Vi. V TAVR or Redo SAVR Deharo et al. , J Am Coll Cardiol 2020; 76: 489
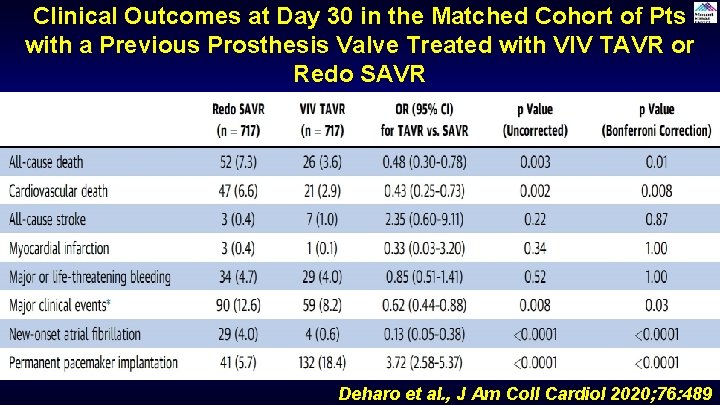
Clinical Outcomes at Day 30 in the Matched Cohort of Pts with a Previous Prosthesis Valve Treated with VIV TAVR or Redo SAVR Deharo et al. , J Am Coll Cardiol 2020; 76: 489
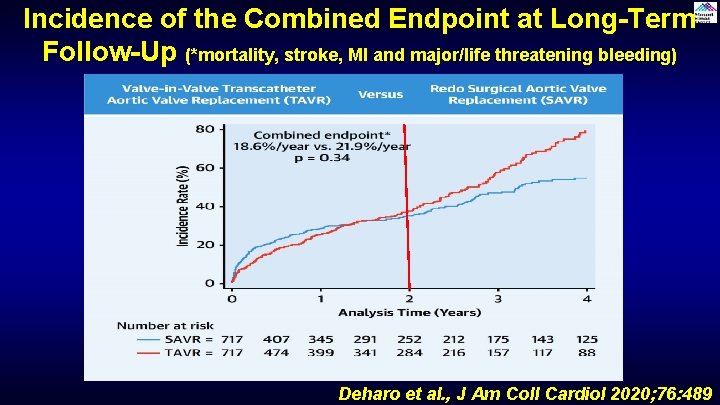
Incidence of the Combined Endpoint at Long-Term Follow-Up (*mortality, stroke, MI and major/life threatening bleeding) Deharo et al. , J Am Coll Cardiol 2020; 76: 489
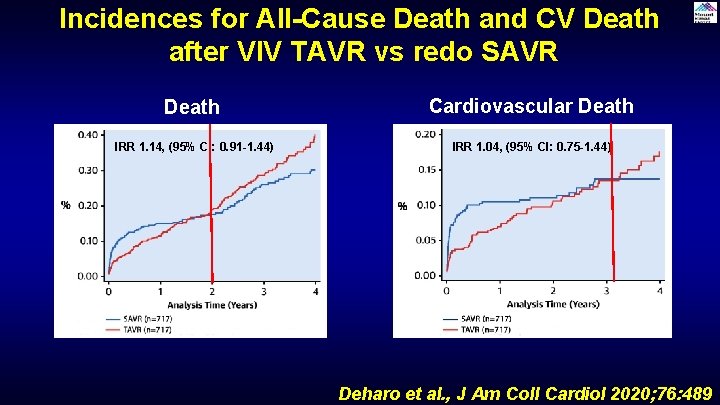
Incidences for All-Cause Death and CV Death after VIV TAVR vs redo SAVR Death Cardiovascular Death IRR 1. 14, (95% CI: 0. 91 -1. 44) IRR 1. 04, (95% CI: 0. 75 -1. 44) Deharo et al. , J Am Coll Cardiol 2020; 76: 489
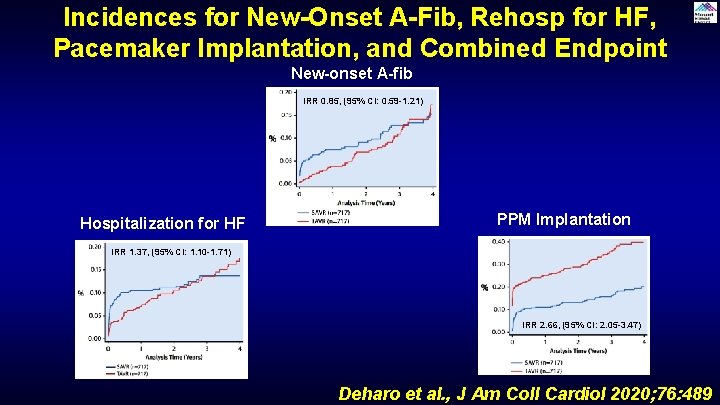
Incidences for New-Onset A-Fib, Rehosp for HF, Pacemaker Implantation, and Combined Endpoint New-onset A-fib IRR 0. 85, (95% CI: 0. 59 -1. 21) Hospitalization for HF PPM Implantation IRR 1. 37, (95% CI: 1. 10 -1. 71) IRR 2. 66, (95% CI: 2. 05 -3. 47) Deharo et al. , J Am Coll Cardiol 2020; 76: 489
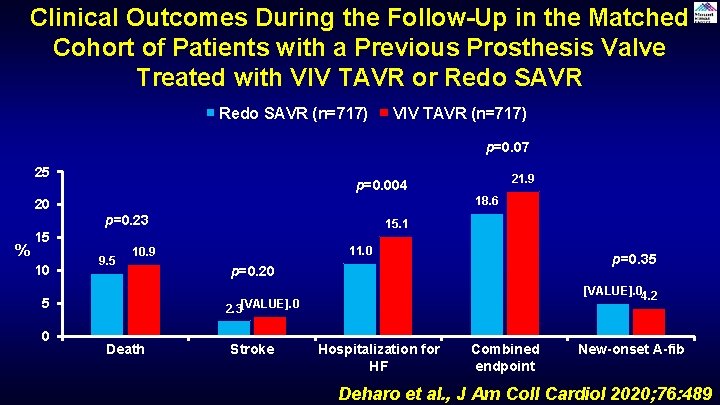
Clinical Outcomes During the Follow-Up in the Matched Cohort of Patients with a Previous Prosthesis Valve Treated with VIV TAVR or Redo SAVR (n=717) VIV TAVR (n=717) p=0. 07 25 20 % 18. 6 p=0. 23 15 10 9. 5 15. 1 11. 0 10. 9 5 0 21. 9 p=0. 004 p=0. 35 p=0. 20 [VALUE]. 04. 2 2. 3[VALUE]. 0 Death Stroke Hospitalization for HF Combined endpoint New-onset A-fib Deharo et al. , J Am Coll Cardiol 2020; 76: 489
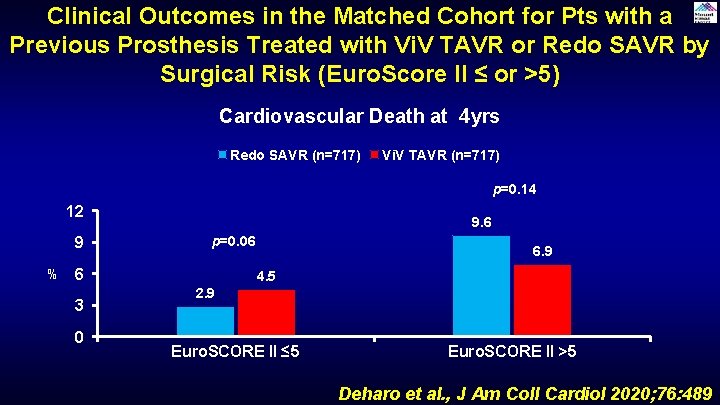
Clinical Outcomes in the Matched Cohort for Pts with a Previous Prosthesis Treated with Vi. V TAVR or Redo SAVR by Surgical Risk (Euro. Score II ≤ or >5) Cardiovascular Death at 4 yrs Redo SAVR (n=717) Vi. V TAVR (n=717) p=0. 14 12 9 % 9. 6 p=0. 06 6 3 0 6. 9 4. 5 2. 9 Euro. SCORE II ≤ 5 Euro. SCORE II >5 Deharo et al. , J Am Coll Cardiol 2020; 76: 489

Although redo SAVR trended better after 2 yrs

We need a RCT of Vi. V TAVR vs redo SAVR in degenerated bio-aortic valve prosthesis
Possible Explanations for Higher F/U mortality after Vi. V TAVR vs SAVR • Increase rates of prosthesis-patient mismatch (PPM) which occurs in about 50 -60% of pts with higher residual trans-aortic gradients • Increased moderate+ paravalvular leaks (PVL) • Increased re-hospitalization rates • Higher need for PPM may result in pacing-induced cardiomyopathy in some cases • Rare cases of valve thrombosis due to aortic sinus flow stasis or delayed coronary obstructions

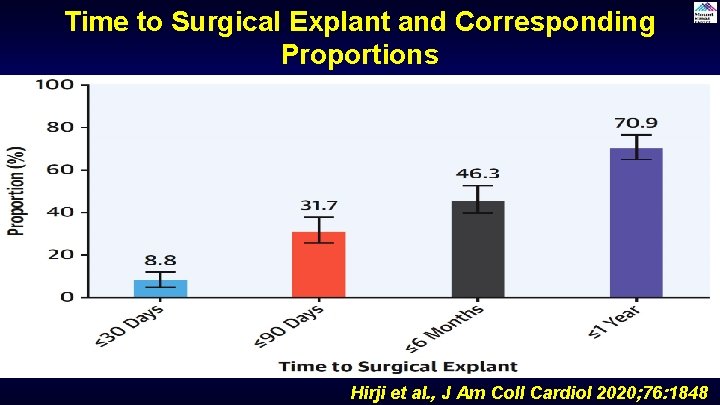
Time to Surgical Explant and Corresponding Proportions Hirji et al. , J Am Coll Cardiol 2020; 76: 1848
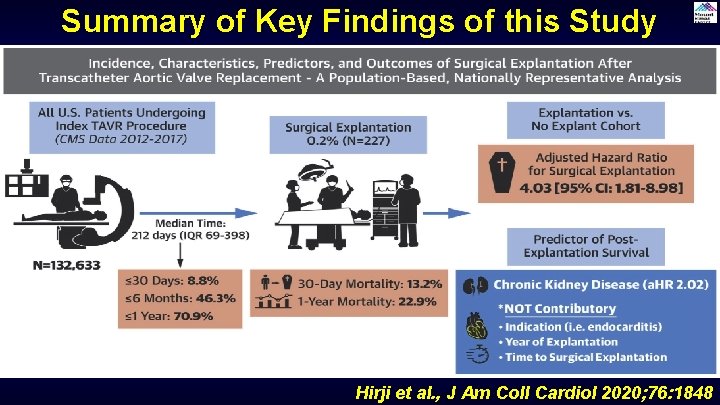
Summary of Key Findings of this Study Hirji et al. , J Am Coll Cardiol 2020; 76: 1848

Take Home Messages Update in Vi. V TAVR vs redo SAVR for bio-AV degeneration • Redo SAVR is associated with higher surgical mortality and hence Vi. V TAVR is increasingly being used to treat bio-prosthetic AV degeneration. Short term outcomes favors Vi. V TAVR vs redo SAVR but long-term outcomes at 4 -5 years are conflicting • A large French registry database reported higher mortality and HF readmission after Vi. V TAVR vs SAVR in a large cohort of propensity matched bio. AV degeneration pts. In contrast, a Canadian registry favored Vi. V TAVR over redo SAVR in a small matched bio. AV degenaration pts. Hence a RCT is clearly needed.

Question # 1 Following are included in the reported ‘Big Five’ complications of TAVR having adverse longterm prognosis except: A. Stroke B. Effective TAVR valve area C. Bleeding D. PVL Correct answer: B
Question # 2 Following is the true statements regarding Vi. V TAVR vs redo SAVR in treatment of bioprosthetic AV degeneration except: A. Vi. V TAVR has higher frequent hospitalization B. Vi. V TAVR consistently has higher long-term mortality C. Vi. V TAVR has higher 30 -day mortality and adverse outcomes D. Vi. V TAVR has higher incidence of coronary obstruction Correct answer: C

Question # 3 Following are the possible explanation for higher adverse long-term outcomes after Vi. V TAVR vs SAVR in treatment on degenerated AV bioprosthesis except: A. Higher PVL B. Higher residual gradient/PPM C. Higher valve thrombosis D. Higher stroke rates Correct answer: D
- Slides: 49