Structural Analysis 1 Adv Higher Unit 3 Topic





- Slides: 19

Structural Analysis 1 Adv Higher Unit 3 Topic 4 Unit 3. 4 Structural Analysis 1

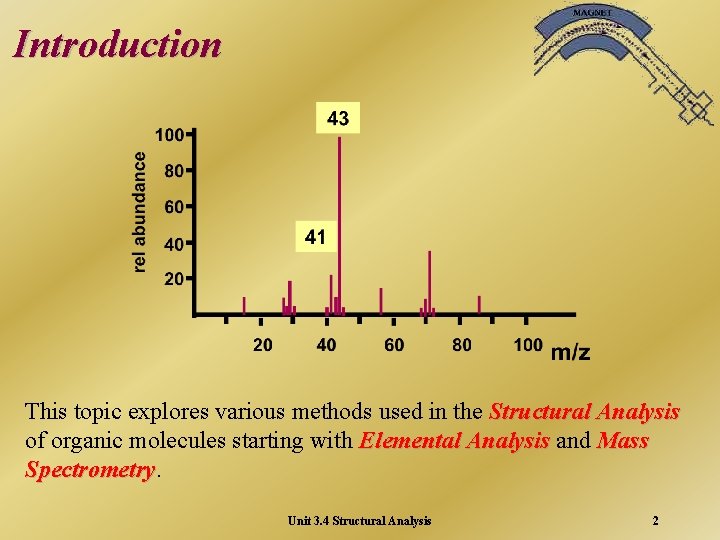
Introduction This topic explores various methods used in the Structural Analysis of organic molecules starting with Elemental Analysis and Mass Spectrometry Unit 3. 4 Structural Analysis 2

Elemental Analysis 1 Empirical formulas are determined by combustion analysis: analysis Carbon, Carbon Hydrogen, Hydrogen Sulphur & Nitrogen can all be determined by combustion analysis Other elements can be determined by other methods. KHS Chemistry Unit 3. 4 Structural Analysis 3

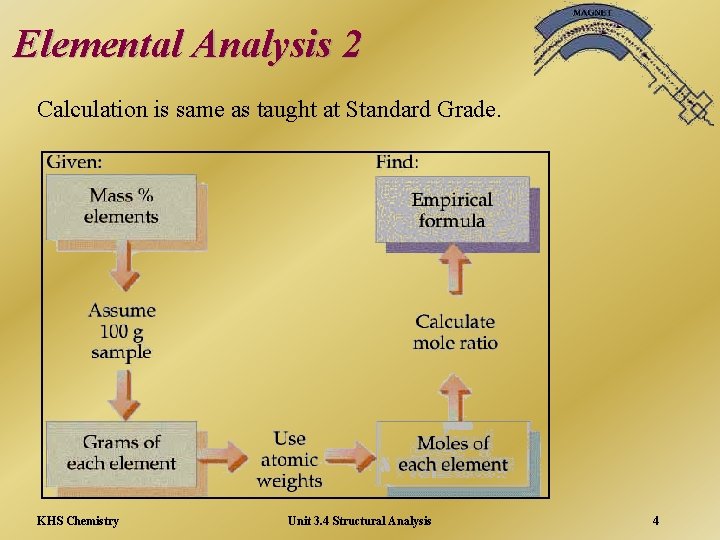
Elemental Analysis 2 Calculation is same as taught at Standard Grade. KHS Chemistry Unit 3. 4 Structural Analysis 4

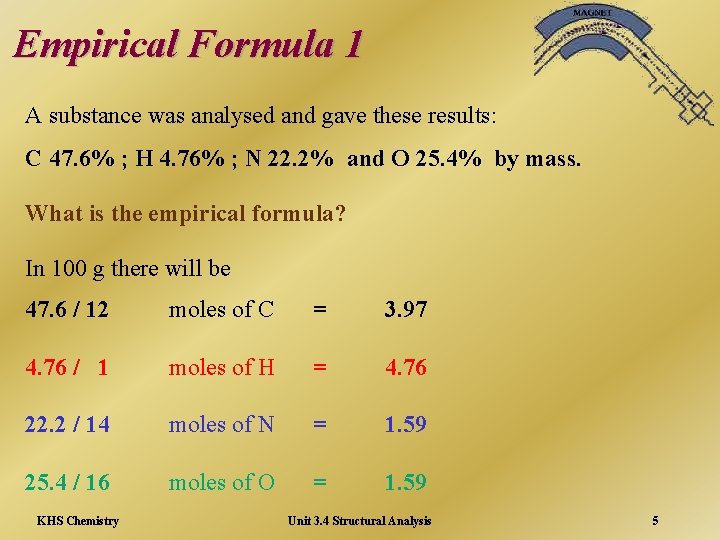
Empirical Formula 1 A substance was analysed and gave these results: C 47. 6% ; H 4. 76% ; N 22. 2% and O 25. 4% by mass. What is the empirical formula? In 100 g there will be 47. 6 / 12 moles of C = 3. 97 4. 76 / 1 moles of H = 4. 76 22. 2 / 14 moles of N = 1. 59 25. 4 / 16 moles of O = 1. 59 KHS Chemistry Unit 3. 4 Structural Analysis 5

Empirical Formula 2 Ratio of C: H: N: O is 3. 97: 4. 76: 1. 59 Divide by smallest number to simplify Simplifying ratio: 2. 5: 2. 99: 1: 1 Whole number ratio is 5: 6: 2: 2 Empirical formula is C 5 H 6 N 2 O 2 KHS Chemistry Unit 3. 4 Structural Analysis 6

Mass Spectrometry A mass spectrometer does three things ¬ vaporises a minute sample of compound (10 -10 g) ionises the vaporised molecules ® separates and analyses the ions, produced when the molecules fall apart, apart according to their mass/charge ratio, giving a mass spectrum KHS Chemistry Unit 3. 4 Structural Analysis 7

Mass Spectrometer KHS Chemistry Unit 3. 4 Structural Analysis 8

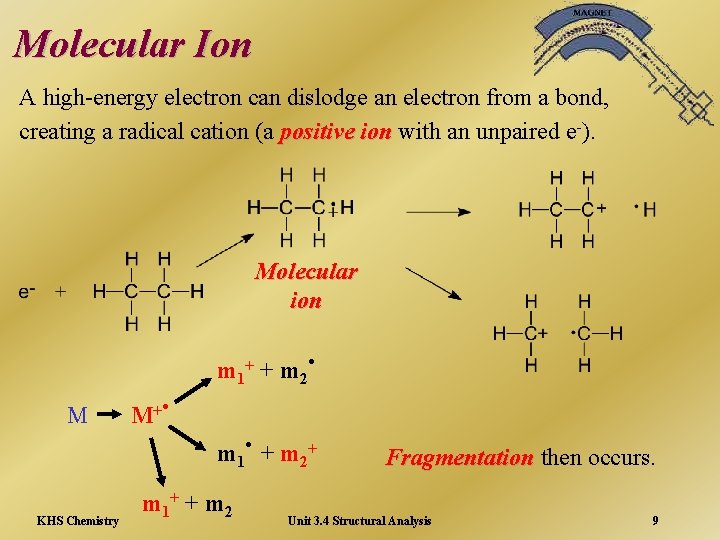
Molecular Ion A high-energy electron can dislodge an electron from a bond, creating a radical cation (a positive ion with an unpaired e-). Molecular ion M M+ . m 1+ 2 . m +m + 1 KHS Chemistry . +m m 1+ + m 2 2 Fragmentation then occurs. Unit 3. 4 Structural Analysis 9

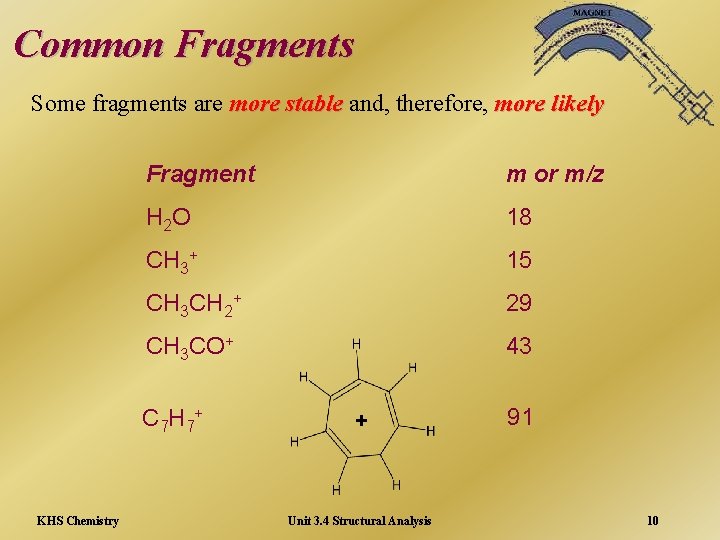
Common Fragments Some fragments are more stable and, therefore, more likely Fragment m or m/z H 2 O 18 CH 3+ 15 CH 3 CH 2+ 29 CH 3 CO+ 43 C 7 H 7+ KHS Chemistry + Unit 3. 4 Structural Analysis 91 10

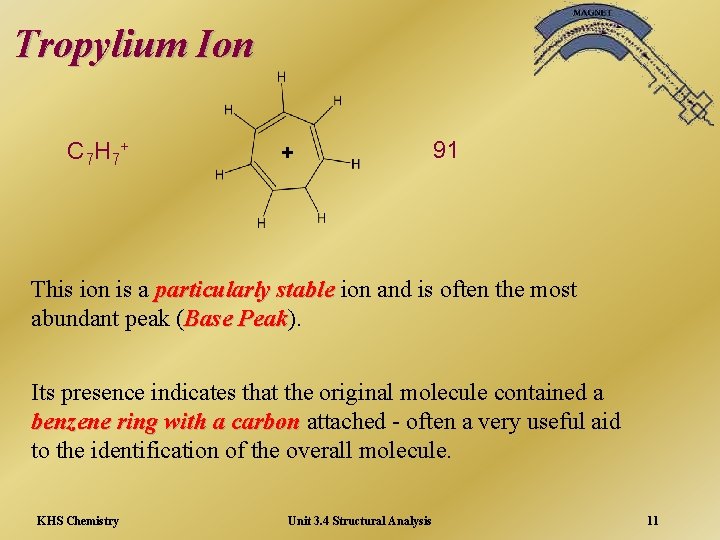
Tropylium Ion C 7 H 7+ + 91 This ion is a particularly stable ion and is often the most abundant peak (Base Peak). Peak Its presence indicates that the original molecule contained a benzene ring with a carbon attached - often a very useful aid to the identification of the overall molecule. KHS Chemistry Unit 3. 4 Structural Analysis 11

The Mass Spectrum Masses are graphed or tabulated according to their relative abundance. KHS Chemistry Unit 3. 4 Structural Analysis 12

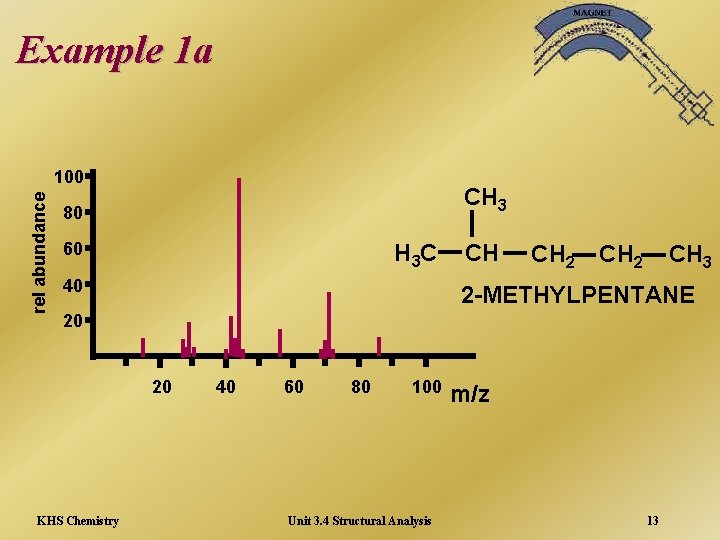
Example 1 a rel abundance 100 CH 3 80 60 H 3 C 40 CH CH 2 CH 3 2 -METHYLPENTANE 20 20 KHS Chemistry 40 60 80 100 Unit 3. 4 Structural Analysis m/z 13

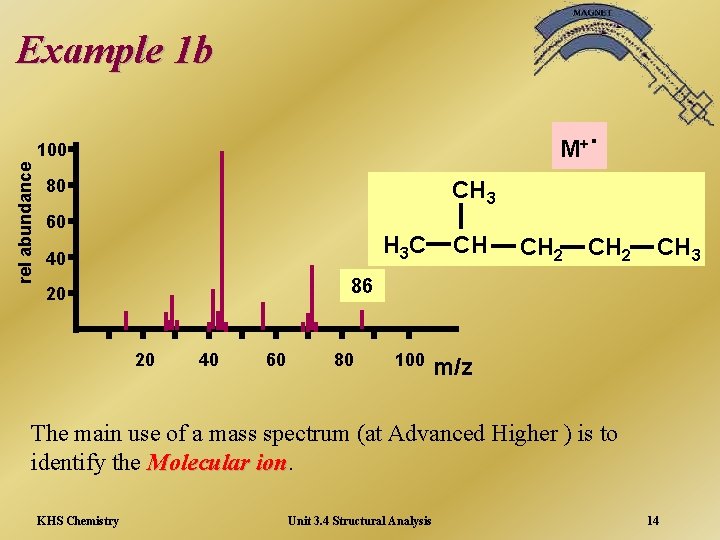
Example 1 b M+ rel abundance 100 80 . CH 3 60 H 3 C 40 CH CH 2 CH 3 86 20 20 40 60 80 100 m/z The main use of a mass spectrum (at Advanced Higher ) is to identify the Molecular ion KHS Chemistry Unit 3. 4 Structural Analysis 14

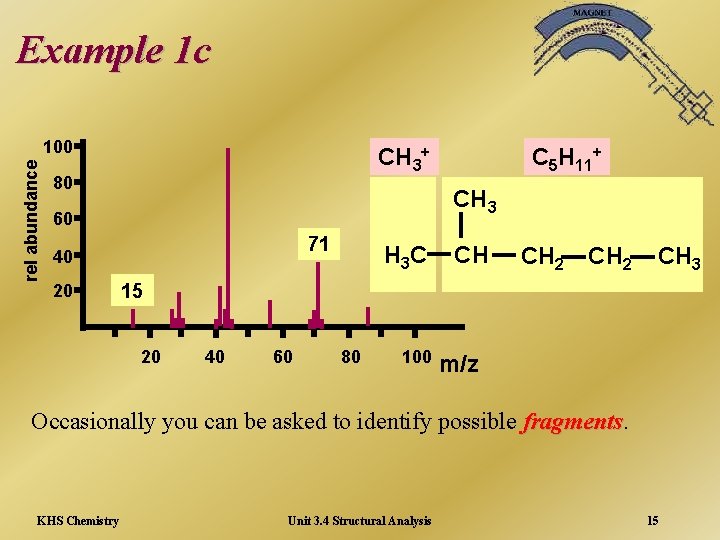
Example 1 c rel abundance 100 CH 3+ 80 CH 3 60 71 40 20 C 5 H 11+ H 3 C CH CH 2 CH 3 15 20 40 60 80 100 m/z Occasionally you can be asked to identify possible fragments KHS Chemistry Unit 3. 4 Structural Analysis 15

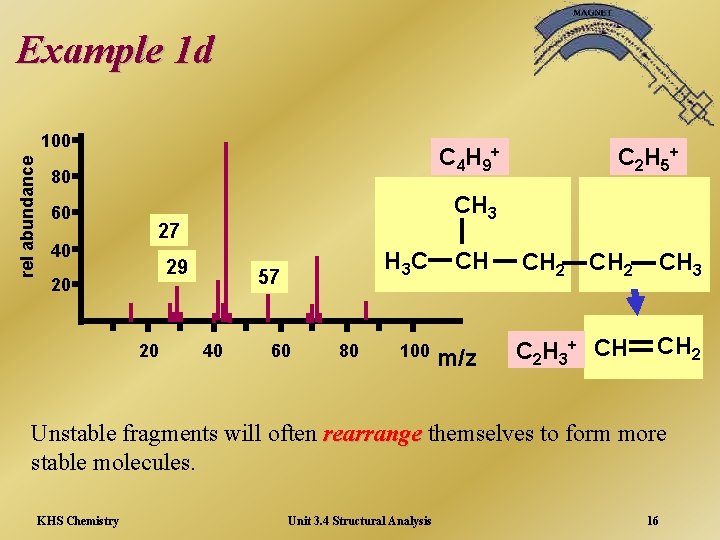
Example 1 d rel abundance 100 C 4 H 9+ 80 60 40 CH 3 27 29 20 20 C 2 H 5+ H 3 C 57 40 60 80 100 CH m/z CH 2 CH 3 C 2 H 3+ CH CH 2 Unstable fragments will often rearrange themselves to form more stable molecules. KHS Chemistry Unit 3. 4 Structural Analysis 16

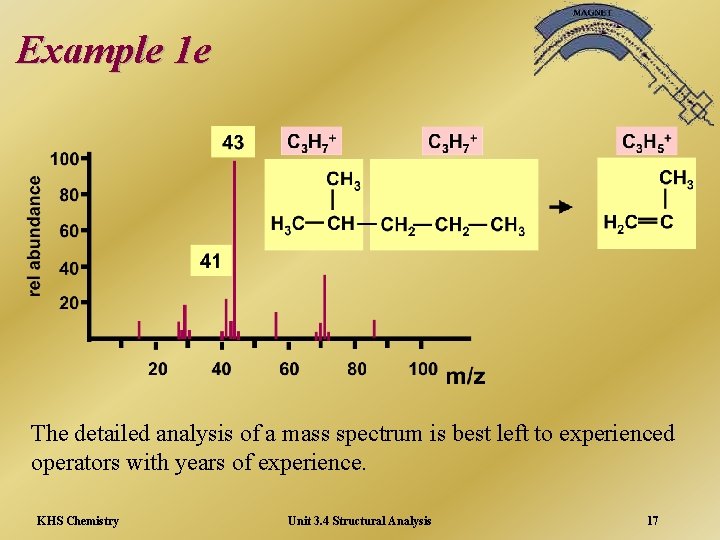
Example 1 e The detailed analysis of a mass spectrum is best left to experienced operators with years of experience. KHS Chemistry Unit 3. 4 Structural Analysis 17

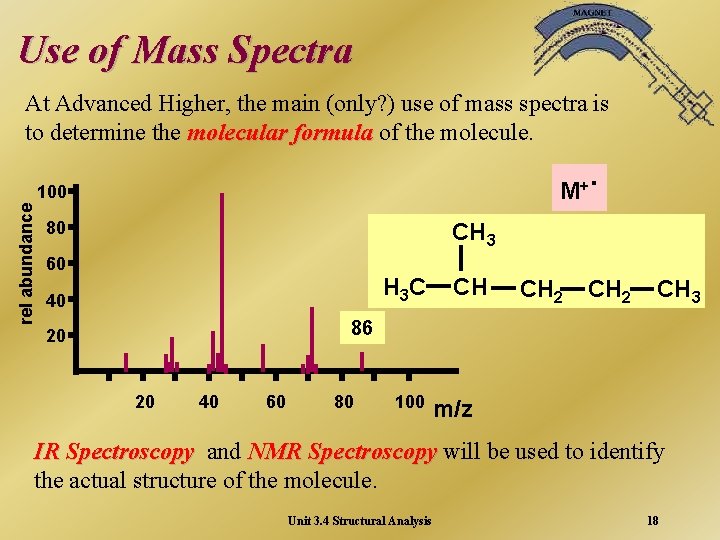
Use of Mass Spectra At Advanced Higher, the main (only? ) use of mass spectra is to determine the molecular formula of the molecule. M+ rel abundance 100 80 . CH 3 60 H 3 C 40 CH CH 2 CH 3 86 20 20 40 60 80 100 m/z IR Spectroscopy and NMR Spectroscopy will be used to identify the actual structure of the molecule. Unit 3. 4 Structural Analysis 18

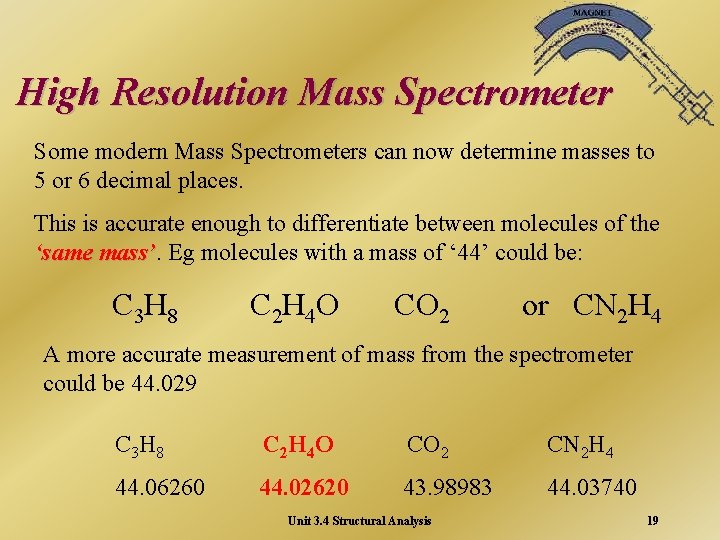
High Resolution Mass Spectrometer Some modern Mass Spectrometers can now determine masses to 5 or 6 decimal places. This is accurate enough to differentiate between molecules of the ‘same mass’ Eg molecules with a mass of ‘ 44’ could be: C 3 H 8 C 2 H 4 O CO 2 or CN 2 H 4 A more accurate measurement of mass from the spectrometer could be 44. 029 C 3 H 8 C 2 H 4 O CO 2 CN 2 H 4 44. 06260 44. 02620 43. 98983 44. 03740 Unit 3. 4 Structural Analysis 19