STRUCTURAL ACTIVITY RELATIONSHIP AMONG THE CHOLINERGIC AND ANTICHOLINERGIC



























































- Slides: 87

STRUCTURAL ACTIVITY RELATIONSHIP AMONG THE CHOLINERGIC AND ANTICHOLINERGIC AGENT

Cholinergic Agent -A cholinergic drug is a drug that acts on the peripheral nervous system, the central nervous system, or both and enhances the effects that are mediated by acetylcholine

-Also known as para sympathomimetic, is any chemical which functions to enhance the effects mediated by acetylcholine in the central nervous system, the peripheral nervous system, or both.

- It is also known as cholinergic agent, para-sympathomimetic drug or cholinergic agonist.

-It works in 2 ways; -1. By acting directly & mimicking the effects of acetylcholine at one or more acetylcholine receptors present in the body.

2. By acting indirectly by blocking/inhibiting the enzyme acetylcholine that is responsible for the degradation/ hydrolysis of acetylcholine

Cholinergic Receptors Two types, determined by: • Location • Action once stimulated

Mechanism of Action -Direct-acting (agonist) –Bind to cholinergic receptors, causing stimulation -Indirect-acting –Inhibit the enzyme “cholinesterase” Indirect-Acting Cholinergic Agents (Cholinesterase Inhibitors

-Reversible –Bind to cholinesterase for a period of minutes to hours -Irreversible –Bind to cholinesterase and form a permanent covalent bond –The body must make new cholinesterase

Drug Effects of Cholinergic Agents 1. Effects seen when the PSNS is stimulated. 2. Stimulate intestine and bladder –Increased gastric secretions –Increased gastrointestinal motility –Increased urinary frequency

3. Stimulate pupil –Constriction (miosis) –Reduced intraocular pressure 4. Increased salivation and sweating

5. Cardiovascular effects –Decreased heart rate –Vasodilation 6. Respiratory effects –Bronchial constriction, narrowed airways

7. At recommended doses, the cholinergics primarily affect the MUSCARINIC receptors. 8. At high doses, cholinergics stimulate the NICOTINIC receptors.

Therapeutic Uses - Direct-Acting Agents • Reduce intraocular pressure • Useful for glaucoma and intraocular surgery -Examples: acetylcholine, carbachol, pilocarpine

- Direct-Acting Agent—bethanechol • Increases tone and motility of bladder and GI tract • Relaxes sphincters in bladder and GI tract, allowing them to empty • Helpful for postsurgical atony of the bladder and GI tract

• Indirect-Acting Agents • Cause skeletal muscle contractions • Used for diagnosis and treatment of myasthenia gravis • Used to reverse neuromuscular blocking agents • Used to reverse anticholinergic poisoning (antidote) Examples: physostigmine, pyridostigmine

- Indirect-Acting Agent—donepezil (Aricept) • Used in the treatment of mild to moderate Alzheimer’s disease. • Helps to increase or maintain memory and learning capabilities

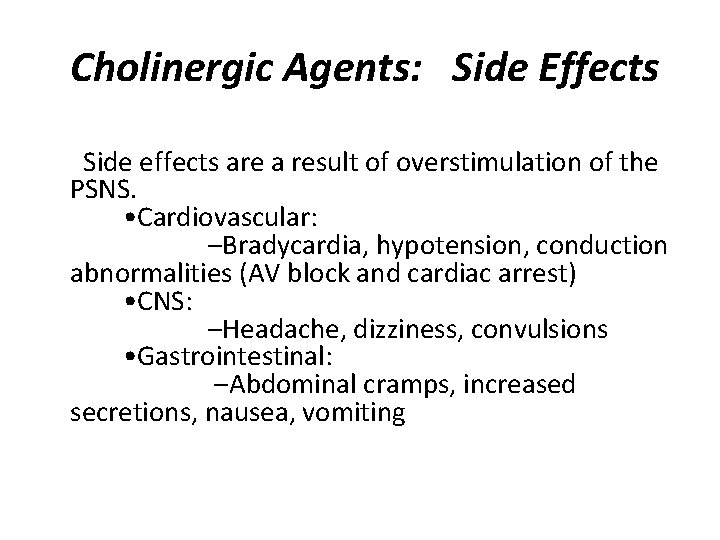
Cholinergic Agents: Side Effects Side effects are a result of overstimulation of the PSNS. • Cardiovascular: –Bradycardia, hypotension, conduction abnormalities (AV block and cardiac arrest) • CNS: –Headache, dizziness, convulsions • Gastrointestinal: –Abdominal cramps, increased secretions, nausea, vomiting

• • Respiratory: –Increased bronchial secretions, bronchospasms • Other: –Lacrimation, sweating, salivation, loss of binocular accommodation, miosis

Drug interactions • Anticholinergics, antihistamines, and sympathomimetics antagonize cholinergic agents, resulting in decreased responses

Cholinergic Blocking Agentsantagonist ( Anticholinergic Agent) -An anticholinergic drug is a drug or an agent that competes with the neurotransmitter "acetylcholine" for its binding sites at synaptic junctions thereby suppressing or inhibiting its activity and thus preventing the transmission of parasympathetic nerve impulses.

-Depending on the type of receptor to act on, anticholinergic drugs are either classified as muscarinic antagonists or nicotinic antagonists.

- They are compounds which prevent acetylcholine from stimulating the receptor site and thus act as antagonists. - Drugs that block or inhibit the actions of acetylcholine (ACh) in the parasympathetic nervous system (PSNS)

Mechanism of Action of Cholinergic Blocking Agents • Competitive antagonists • Compete with ACh • Block ACh at the muscarinic receptors in the PSNS –As a result, ACh is unable to bind to the receptor site and cause a cholinergic effect. • Once these drugs bind to receptors, they inhibit nerve transmission at these receptors.

Chemical class of Cholinergic Blocking Agents - Natural 1. atropine 2. hyoscyamine 3. scopolamine 4. belladonna

Synthetic/Semisynthetic • • anisotropine clidinium • dicyclomine glycopyrrolate • hexocyclium homatropine • ipratropium isopropamide • oxybutynin propantheline • • tolterodine tridihexethyl

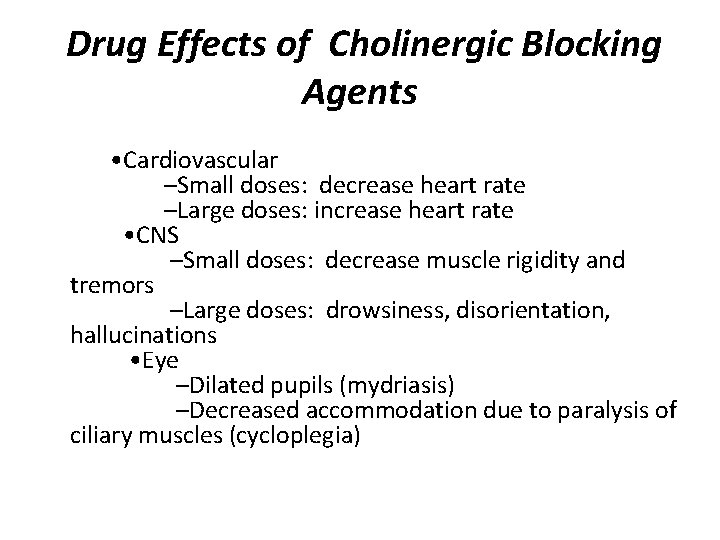
Drug Effects of Cholinergic Blocking Agents • Cardiovascular –Small doses: decrease heart rate –Large doses: increase heart rate • CNS –Small doses: decrease muscle rigidity and tremors –Large doses: drowsiness, disorientation, hallucinations • Eye –Dilated pupils (mydriasis) –Decreased accommodation due to paralysis of ciliary muscles (cycloplegia)

• Gastrointestinal –Relax smooth muscle tone of GI tract –Decrease intestinal and gastric secretions –Decrease motility and peristalsis • Genitourinary –Relaxed detrusor muscle –Increased constriction of internal sphincter –Result: urinary retention

• Glandular –Decreased bronchial secretions, salivation, sweating • Respiratory –Decreased bronchial secretions –Dilated bronchial airways

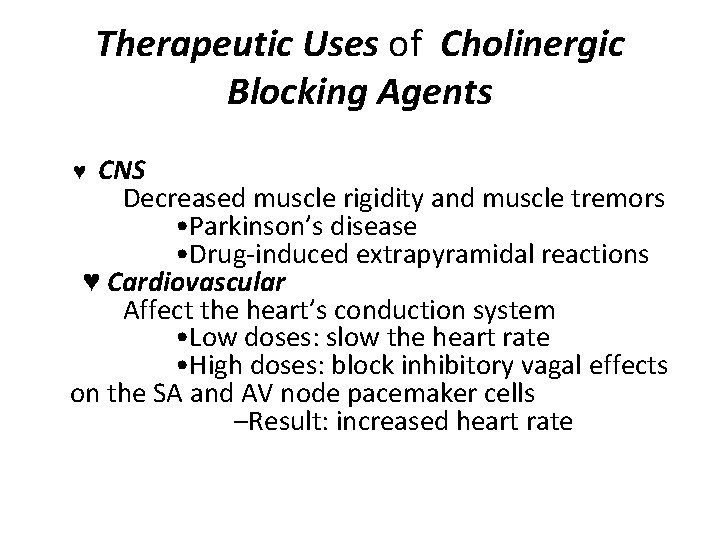
Therapeutic Uses of Cholinergic Blocking Agents ♥ CNS Decreased muscle rigidity and muscle tremors • Parkinson’s disease • Drug-induced extrapyramidal reactions ♥ Cardiovascular Affect the heart’s conduction system • Low doses: slow the heart rate • High doses: block inhibitory vagal effects on the SA and AV node pacemaker cells –Result: increased heart rate

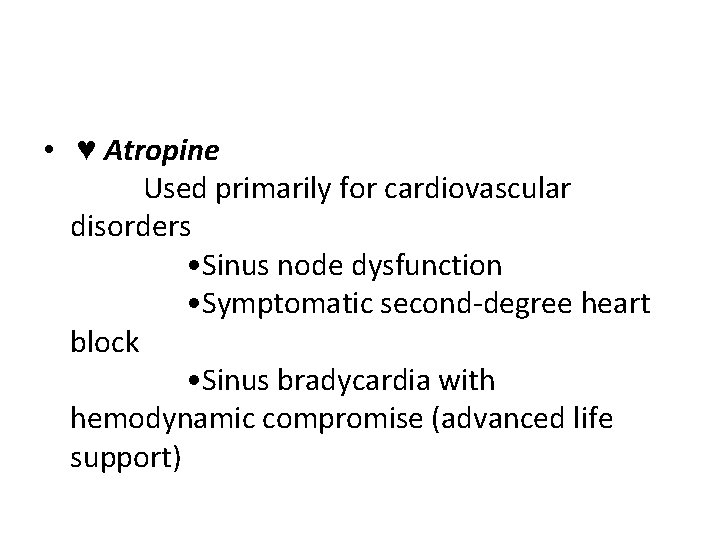
• ♥ Atropine Used primarily for cardiovascular disorders • Sinus node dysfunction • Symptomatic second-degree heart block • Sinus bradycardia with hemodynamic compromise (advanced life support)

♥ Respiratory Blocking the cholinergic stimulation of the PSNS allows unopposed action of the SNS. • Results: –Decreased secretions from nose, mouth, pharynx, bronchi –Relaxed smooth muscles in bronchi and bronchioles –Decreased airway resistance –Bronchodilation Respiratory agents are used to treat: • Exercise-induced bronchospasms • Chronic bronchitis • Asthma • Chronic obstructive pulmonary disease

• ♥ Gastrointestinal PSNS controls gastric secretions and smooth muscles that produce gastric motility. • Blockade of PSNS results in: –Decreased secretions –Relaxation of smooth muscle –Decreased GI motility and peristalsis Gastrointestinal agents are used to treat: • Peptic ulcer disease • Irritable bowel disease • GI hypersecretory states

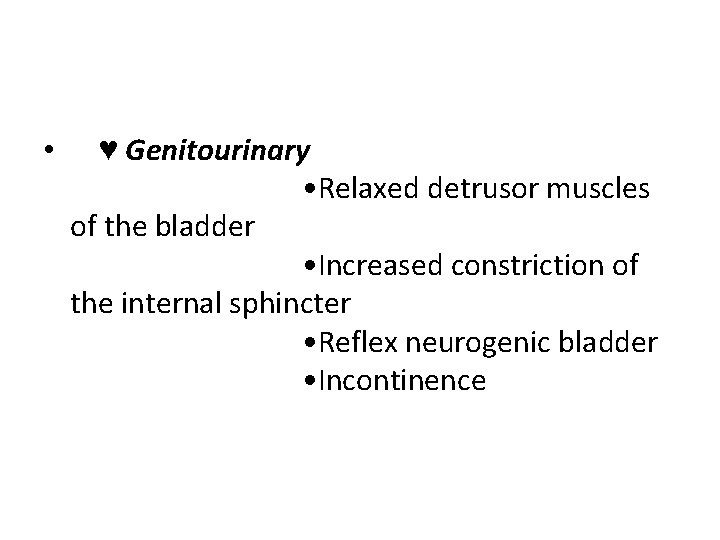
• ♥ Genitourinary • Relaxed detrusor muscles of the bladder • Increased constriction of the internal sphincter • Reflex neurogenic bladder • Incontinence

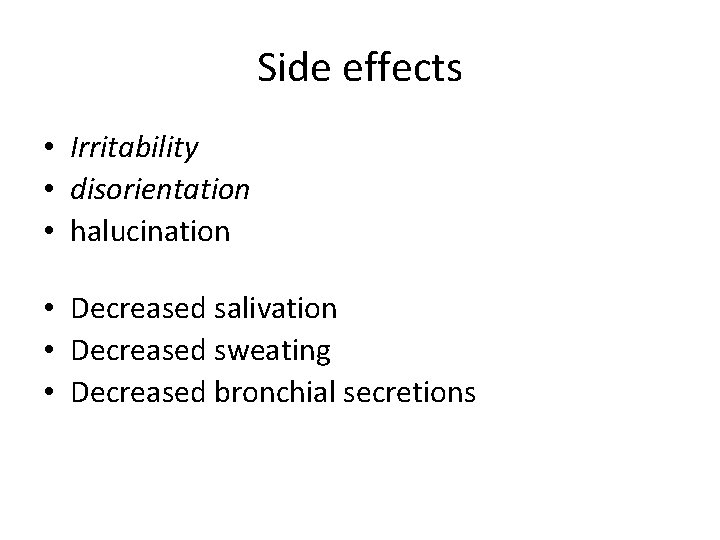
Side effects • Irritability • disorientation • halucination • Decreased salivation • Decreased sweating • Decreased bronchial secretions

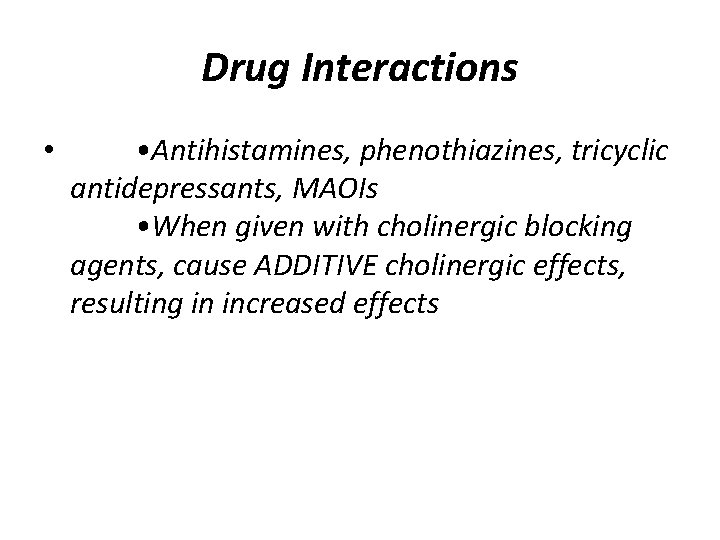
Drug Interactions • • Antihistamines, phenothiazines, tricyclic antidepressants, MAOIs • When given with cholinergic blocking agents, cause ADDITIVE cholinergic effects, resulting in increased effects

STRUCTURAL ACTIVITY RELATIONSHIP

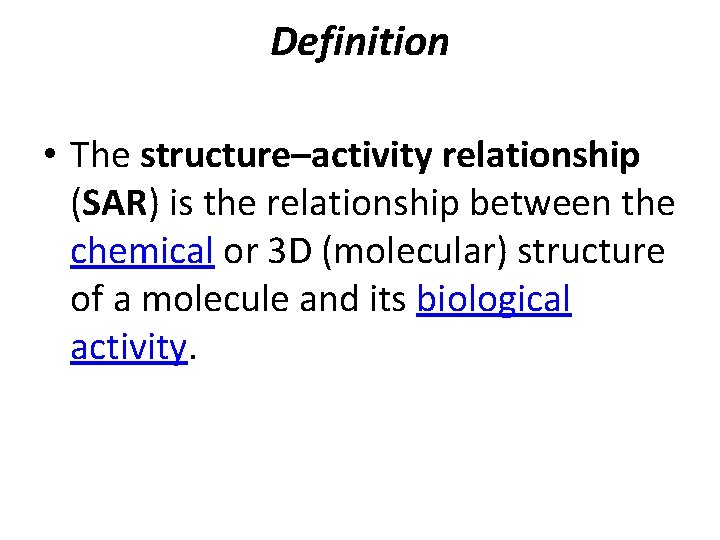
Definition • The structure–activity relationship (SAR) is the relationship between the chemical or 3 D (molecular) structure of a molecule and its biological activity.

• Structure-activity relationship is the relationship between chemical structure and pharmacological activity for a series of compounds.

• SARS is the study of relationship between a drug’s molecular structure and the drug’s biological activity.

• The Structure-activity relationship (SAR) is a means by which the effect of a drug or toxic chemical on an animal, plant or the environment can be related to its molecular structure.

• This type of relationship may be assessed by considering a series of molecules and making gradual changes to them, noting the effect upon their biological activity of each change.

• The rationale behind SAR is that the structure of the chemical implicitly determines its physical and biological properties and reactivity, which in interaction with a biological system, determine its biological / toxicological properties.

• Therefore, the science of SAR is in relating the structure to activity, i. e. identifying the key aspects of structure, pertaining to the molecular event(s) in the mechanism of action for the chemical or biological actions of interest.

Pharmacological Effects Of Sars • 1. SARS have made it possible to map out the receptor and prepare a model that can account for the affinity and the intrinsic activity of cholinergic agonist and anticholinergic.

2. The analysis of SAR enables the determination of the chemical group responsible for evoking a target biological effect in the organism

• 3. Structure-activity studies are critical to designing a pharmaceutical with the greatest potency and least side effects.

HISTORY • Brown and Fraser in 1869 showed that many compounds containing tertiary amine groups became muscle relaxants when converted to quaternary ammonium compounds.

• This hypothesis was later rejected. “Molecules that block the effects of natural neurotransmitters (antagonists) generally are larger in size than the native compound.

Features Of SAR of Cholinergics • A molecule must possess a nitrogen atom capable of bearing a positive charge, preferably a quaternary ammonium salt. • For maximum potency, the size of the alkyl groups substituted on the nitrogen should not exceed the size of a methyl group.

• The molecule should have an oxygen atom, preferably an ester-like oxygen capable of participating in a hydrogen bond. • A two-carbon unit should occur between the oxygen atom and the nitrogen atom.
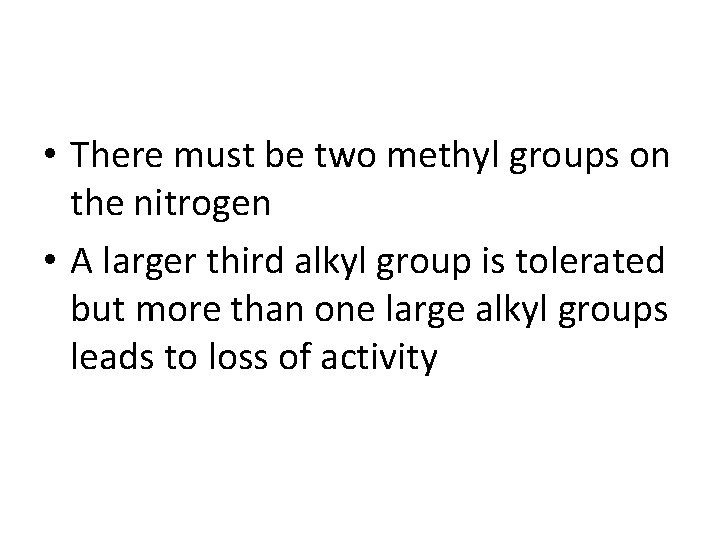
• There must be two methyl groups on the nitrogen • A larger third alkyl group is tolerated but more than one large alkyl groups leads to loss of activity

• There should be no more than five atoms between the nitrogen and the terminal hydrogen for muscarinic (or cholinergic) activity; for maximum potency, the size of the alkyl groups substituted on the nitrogen should not exceed the size of a methyl group;

• The molecule should have an oxygen atom, preferably an ester-like oxygen capable of participating in a hydrogen bond; • There should be a two-carbon unit between the oxygen atom and the nitrogen atom.

STRUCTURAL ACTIVITY OF ANTICHOLINERGICS • With respect to the acid moiety of the anticholinergics, the following structure-activity relationships are found:

• 1. As R 1 is increased from methyl to higher alkyls or becomes hydrogen, alkenyl, amino, or aminoalkyl, psychotropic potency diminishes without much effect on peripheral anticholinergic action.

• 2. R 2 should be an unsubstituted phenyl group, wherase R 3 must be either a cycloalkyl, alkynyl, thienyl, or unsubstituted phenyl. Alkyl, aryl, halide, or hydroxyl substituents on the phenyl rings abolish central action and diminish anticholinergic potency. R 2 and R 3 can also be replaced by hexahydrofluorenyl

• 3. The position of the ester side chain affects central, but not peripheral action, with the 4 piperidyl ester being most potent, the 3 -ester second most and the 2 -ester least.

• 4. R 4 must be a hydroxyl group, whereas compounds with hydrogen or an isosteric methyl group are devoid of central action and have diminished anticholinergic action. If R 4 is hydrogen and the hydroxyl group is present on phenyl, central potency is retained.

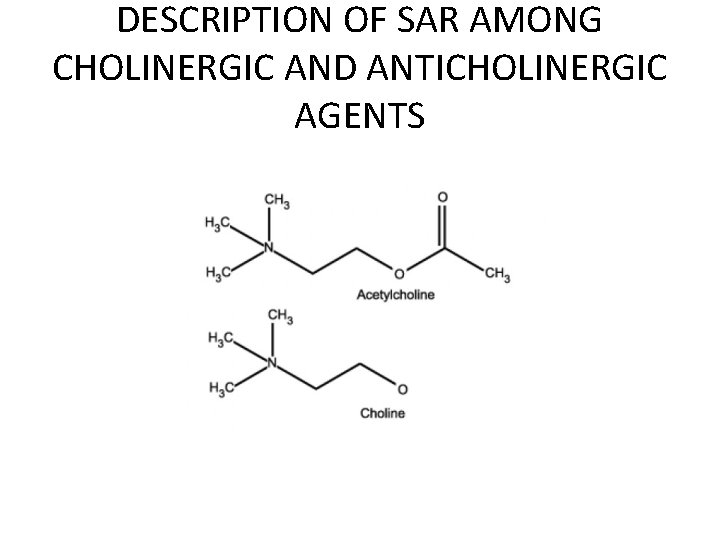
DESCRIPTION OF SAR AMONG CHOLINERGIC AND ANTICHOLINERGIC AGENTS

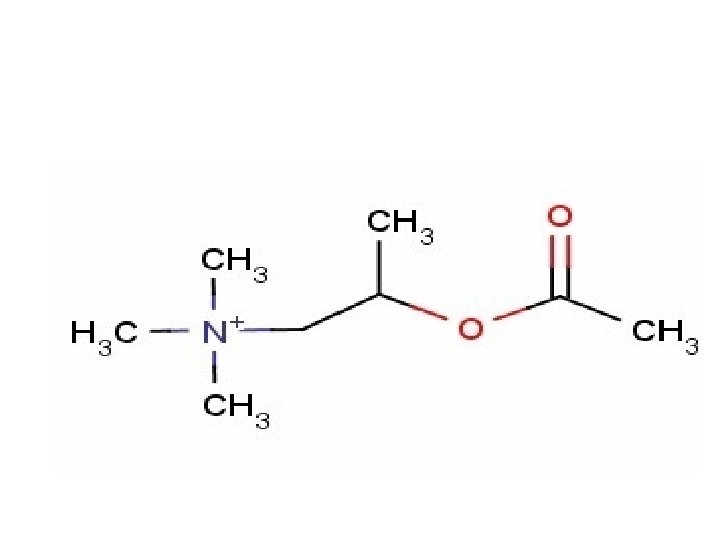
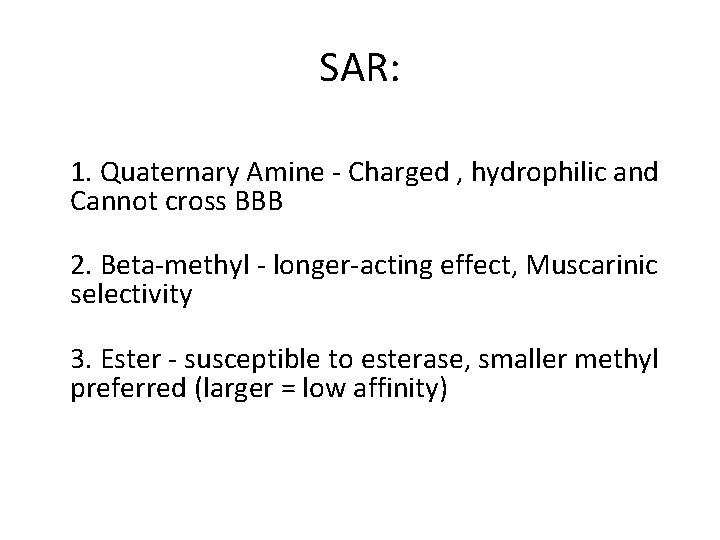
1) Methacholine Name: Methacholine Type of Agent: Direct Muscarinic Agonist


SAR: 1. Quaternary Amine - Charged , hydrophilic and Cannot cross BBB 2. Beta-methyl - longer-acting effect, Muscarinic selectivity 3. Ester - susceptible to esterase, smaller methyl preferred (larger = low affinity)

Indications: • Diagnose bronchial hyperactivity ONLY - Patient inhales aerosolized Methacholine (broncoconstriction) Should NOT be used as a therapeutic agent

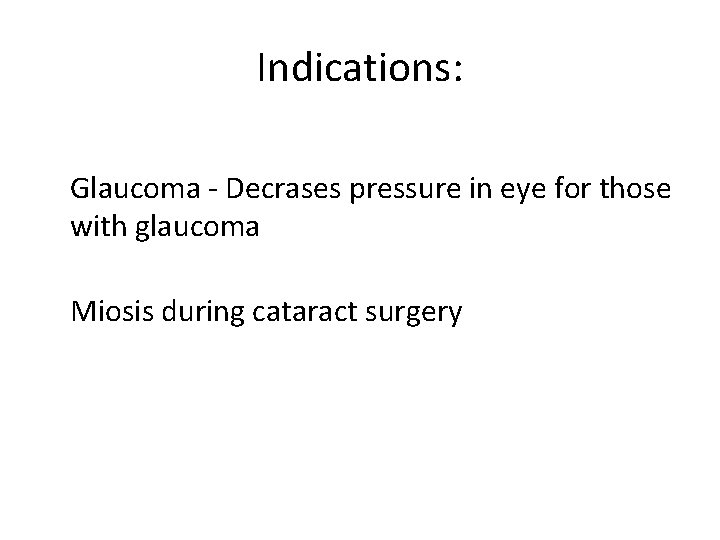
2) Carbachol • Name: Carbamolycholine (Carbachol) Type of Agent: Direct Muscarinic Agonist

SAR: 1. Quaternary Amine - Charged ∴ Hydrophilic, cannot cross BBB 2. Carbamate - Slows down hydrolysis (longlasting) 3. Hydrogen bonding

Indications: Glaucoma - Decrases pressure in eye for those with glaucoma Miosis during cataract surgery

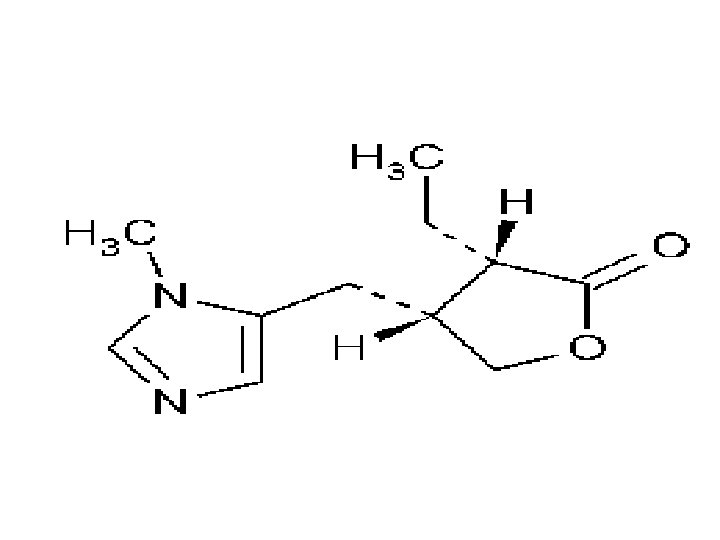
3) Pilocaripine Name: Pilocaripine Type of Agent: Direct Muscarinic Agonist


SAR: 1. Chiral Carbon - allows epimerization, allows for substiuents to change sterochemistry, can be kept refrigerated to avoid epimerization

Indications: Glaucoma Dry mouth (after radiation) Adverse Effects: Visual changes

5) Neostigmine Name: Neostigmine Type of Agent: Indirect Acting ACh. E inhibitor

SAR: • Quaternary Amine - charged ∴ hydrophilic, can't cross BBB Carbamate - slows down hydrolysis ∴ long lasting Hydrogen bonding

Indications: • Myasthenia Gravis - Decrease in release of ACh and less n. ACh receptors Urinary retention Reverse neuromuscular blockage

Adverse Effects: • Allergenic cross-reactivity for Ch. E inhibitors Contraindications Hypersensitivity

7) : Physostigme • Name: Physostigme Type of Agent: Indirect Acting ACh. E inhibitor SAR: Carbamate - slows down hydrolysis ∴ long-acting Hydrogen Bonding

Indications: • Glaucoma Reversal of CNS anticholinergic syndrome

Adverse Effects: • Contraindications GI obstruction Asthma Diabetes CV disease Coadministration of choline esters and depolarizing neuromuscular-blocking agents

8) SCOPOLAMINE Name: Scopolamine (Isopto Hyoscine) Type of Agent: Muscarinic Antagonist SAR: Acyloxy - ethylene - quaternary amine

• Indications: Transdermal - prevent N/V Adverse Effects: Anaphylaxis Bradycardia CNS effects Visual disturbances

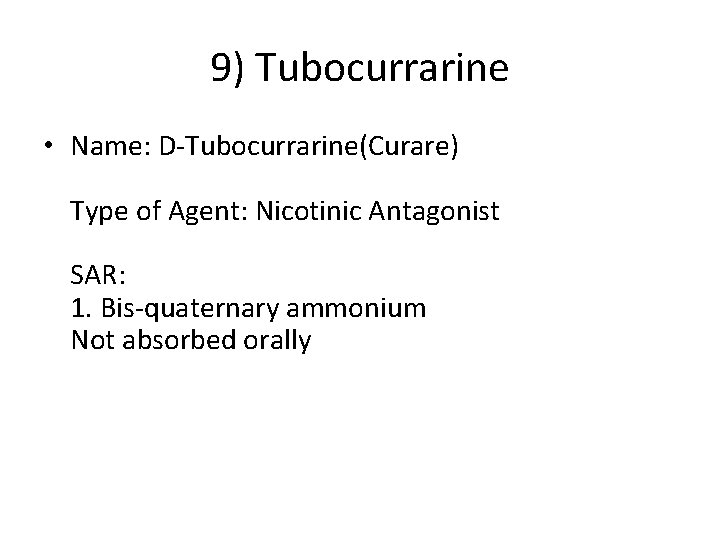
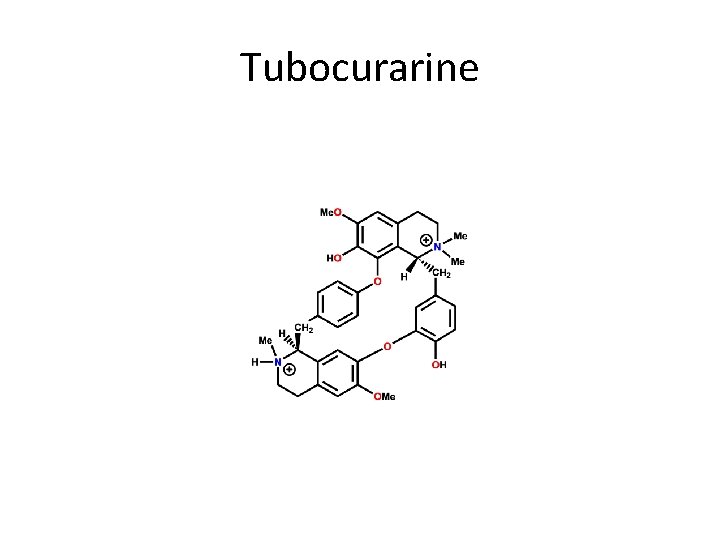
9) Tubocurrarine • Name: D-Tubocurrarine(Curare) Type of Agent: Nicotinic Antagonist SAR: 1. Bis-quaternary ammonium Not absorbed orally

• Indications: nondepolarizing blocker - Death occurs through respiratory paralysis Muscle relaxant during surgery Adverse Effects: Trigger histamine release - BP drops

Tubocurarine

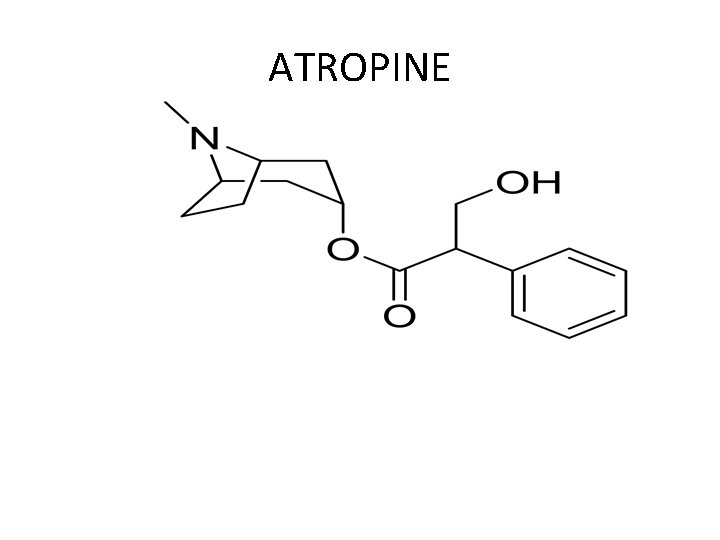
ATROPINE Name: Atropine (Isopto Atropine) Type of Agent: Muscarinic Antagonist SAR: Acyloxy - Ethylene - Quaternary Amine Chiral Carbon, Diasteromeric mixture L-isomer is physiologically active

ATROPINE

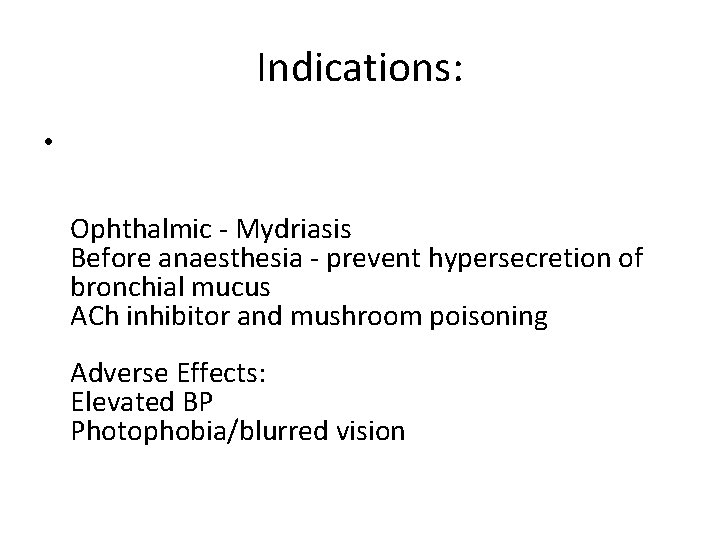
Indications: • Ophthalmic - Mydriasis Before anaesthesia - prevent hypersecretion of bronchial mucus ACh inhibitor and mushroom poisoning Adverse Effects: Elevated BP Photophobia/blurred vision

PRACTISE QUESTIONS • a. Describe the structure-activity relationship of TWO anticholinergic drugs. • b. Compare and contrast the pharmacokinetics and pharmacodynamics of atropine and pilocarpine. •