STRONTIUM ISOTOPES Why and how it is used





















- Slides: 62

STRONTIUM ISOTOPES Why and how it is used in estimating stratigraphic position of carbonates and evaporites Review by B. C. Schreiber

ONE OF THE MOST USEFUL, CONSERVATIVE, AND STABLE ELEMENTS FOR ISOTOPIC STUDY IN SEDIMENTARY ROCKS (CARBONATES AND EVAPORITES) IT HAS BEEN TREATED AS A GEOLOGICAL “TRACER”

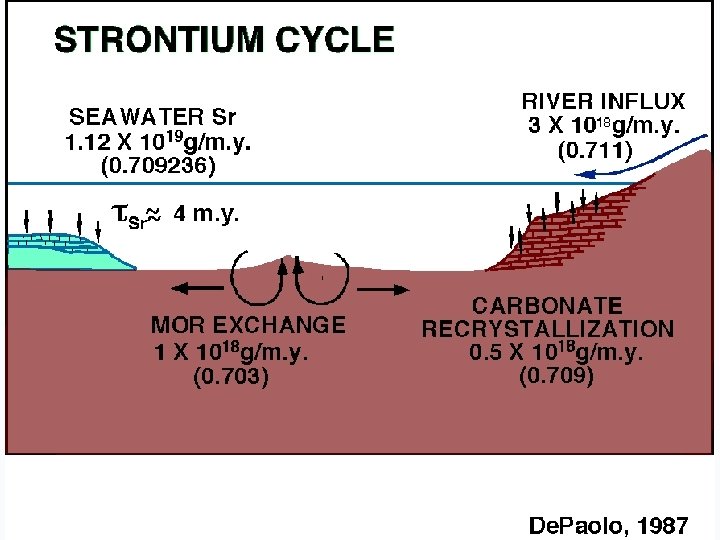
RESIDENCE TIME OF STRONTIUM IN THE OCEANS >2 M. Y. MIXING TIME OF STRONTIUM IN THE OCEANS ~103 YEARS

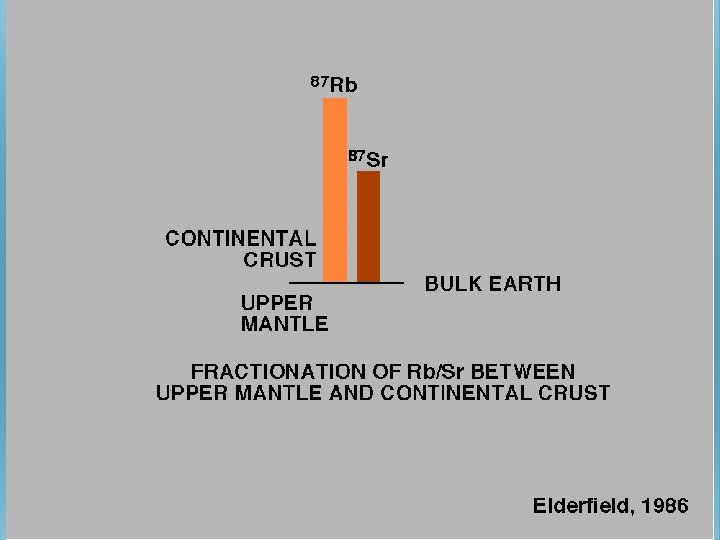
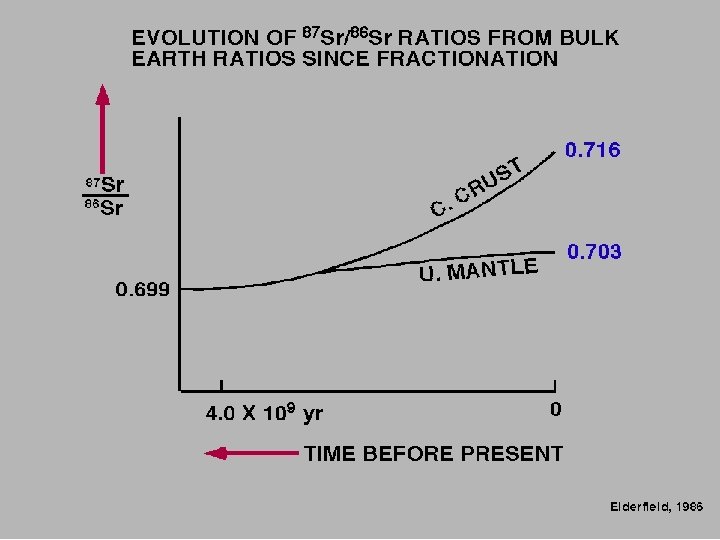
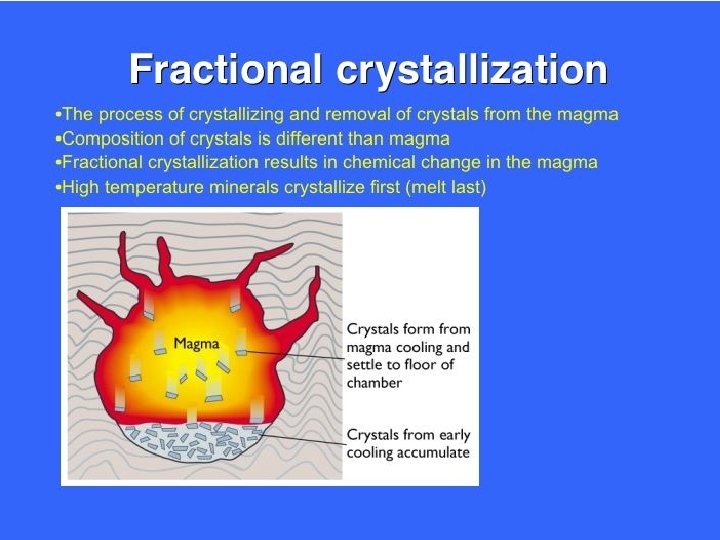
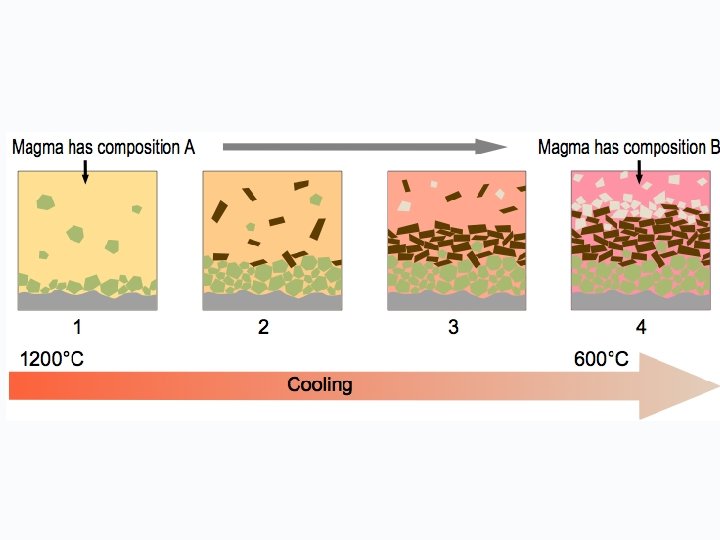
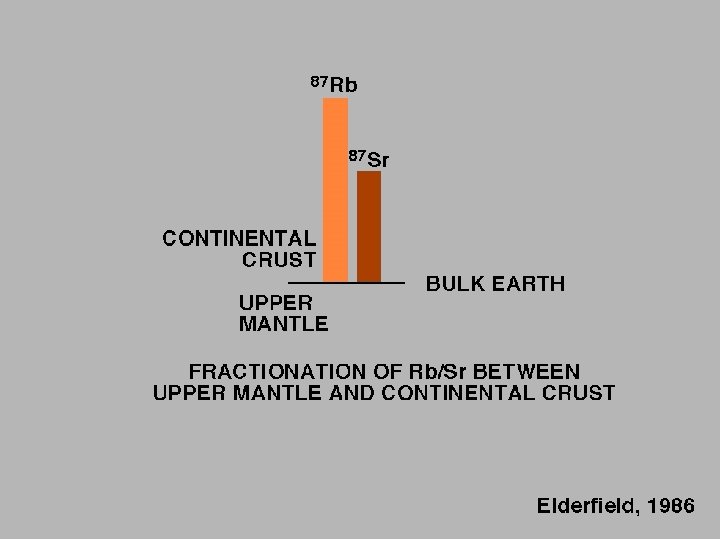
BACKGROUND During fractional crystallization, Sr tends to be come concentrated in the first minerals to crystallize, leaving Rb in the liquid phase. Hence, the Rb/Sr ratio in residual magma may increase over time, resulting in rocks with increasing Rb/Sr ratios with increasing differentiation. Highest ratios occur in pegmatites. Typically, Rb/Sr increases in the order plagioclase, hornblende, K-feldspar, biotite, muscovite. Therefore, given sufficient time for significant production (ingrowth) of radiogenic 87 Sr, measured 87 Sr/86 Sr values will be different in the minerals, increasing in the same order. The Rb-Sr dating method has been used extensively in dating rocks. If the initial amount of Sr is known or can be extrapolated, the age can be determined by measurement of the Rb and Sr concentrations and 87 Sr/86 Sr ratio. The dates indicate the true age of the minerals only if the rocks have not been subsequently altered.

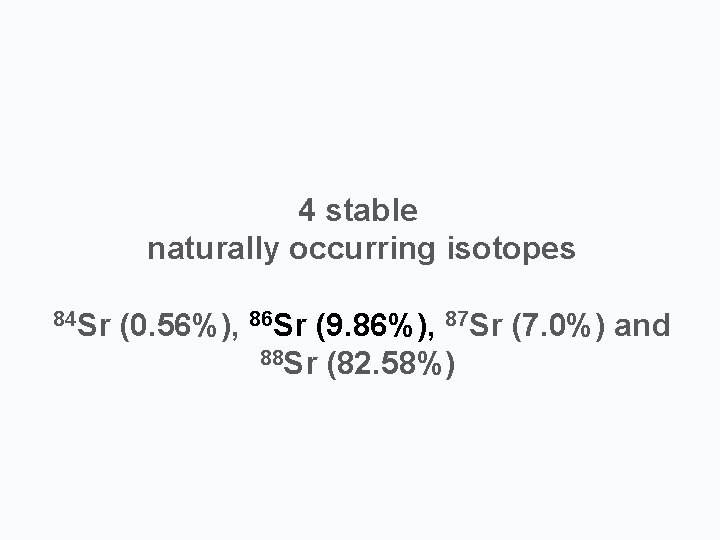
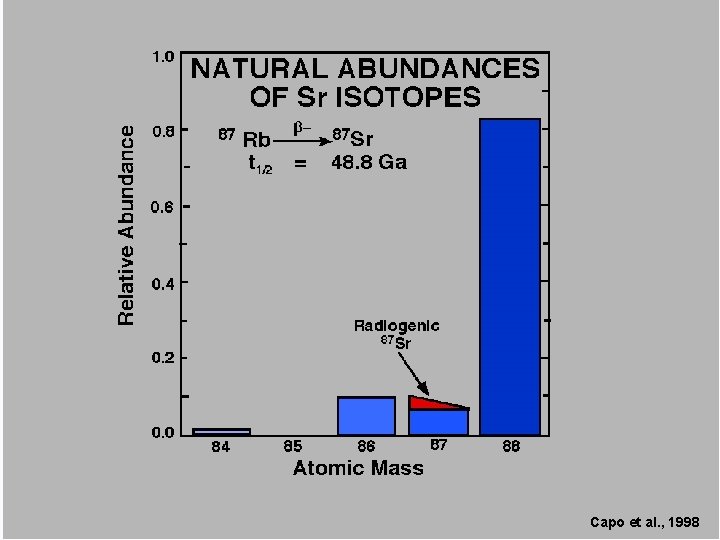
44 stable naturally occurring isotopes 84 84 Sr 86 Sr 87 Sr Sr (0. 56%), 86 Sr (9. 86%), 81 Sr (7. 0%) and 88 88 Sr Sr(82. 58%)

Strontium is present as a ubiquitous minor element in the crust of the Earth –

Strontium is present as a ubiquitous minor element in the crust of the Earth – Present in many rock types.

Strontium is present as a ubiquitous minor element in the crust of the Earth – Present in many rock types. Typically found in concentrations of a few hundred parts per million

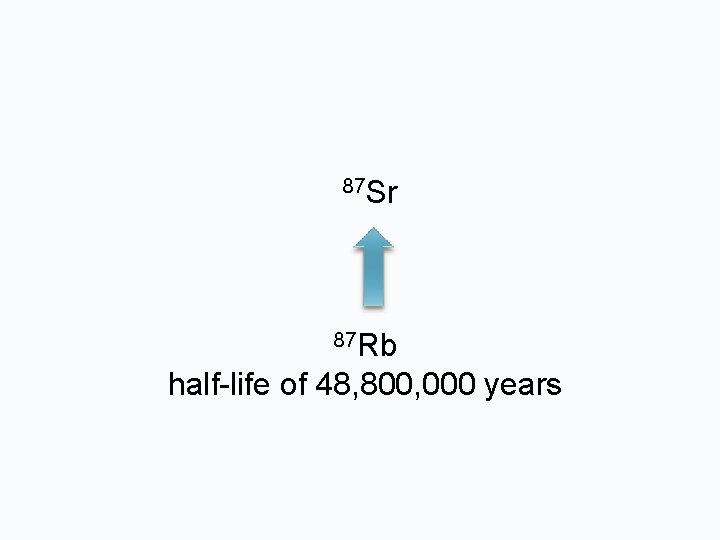
87 Sr 87 Rb half-life of 48, 800, 000 years



44 stable naturally occurring isotopes 84 84 Sr 86 Sr 87 Sr Sr (0. 56%), 86 Sr (9. 86%), 81 Sr (7. 0%) and 88 88 Sr Sr(82. 58%)

4 stable naturally occurring isotopes 84 Sr (0. 56%), 86 Sr (9. 86%), 87 Sr (7. 0%) and 88 Sr (82. 58%)

Capo et al. , 1998

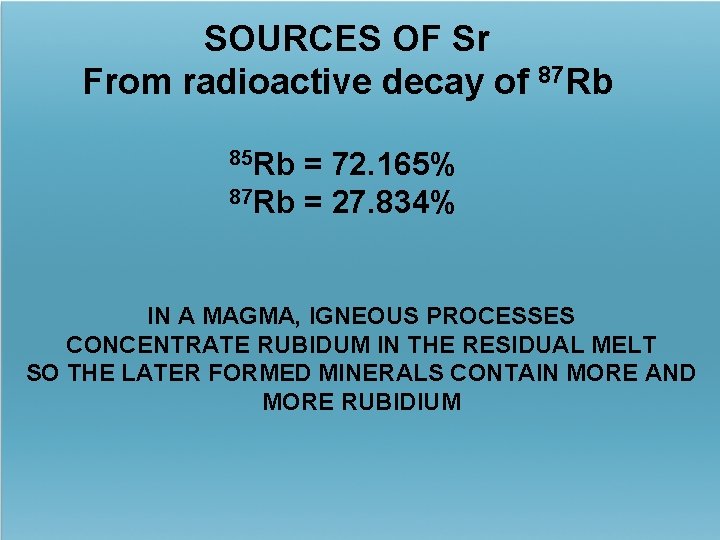
SOURCES OF Sr From radioactive decay of 87 Rb 85 Rb = 72. 165% 87 Rb = 27. 834% IN A MAGMA, IGNEOUS PROCESSES CONCENTRATE RUBIDUM IN THE RESIDUAL MELT SO THE LATER FORMED MINERALS CONTAIN MORE AND MORE RUBIDIUM

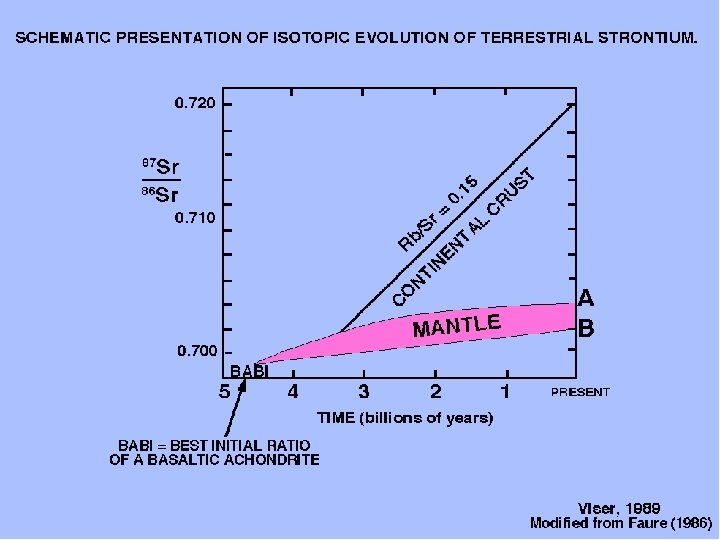
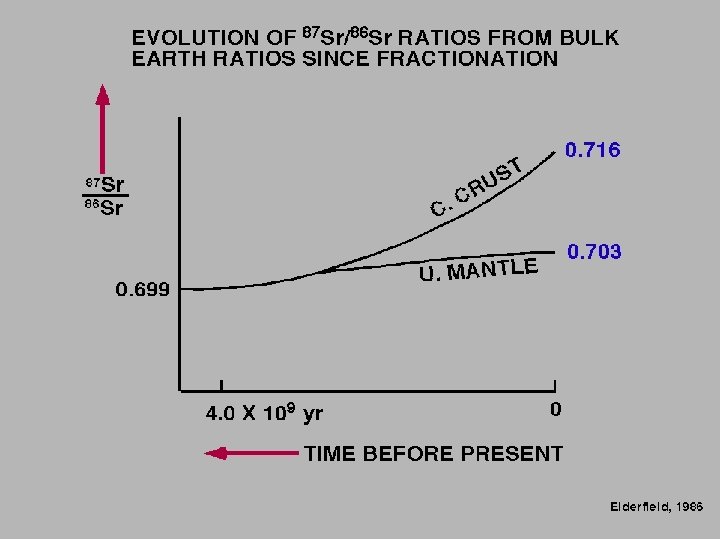
Primordial 87 Sr/86 Sr ratio of 0. 699, value derived from meteorites, NOW HIGHER ON EARTH due to the decay of 87 Rb Faure, 1991


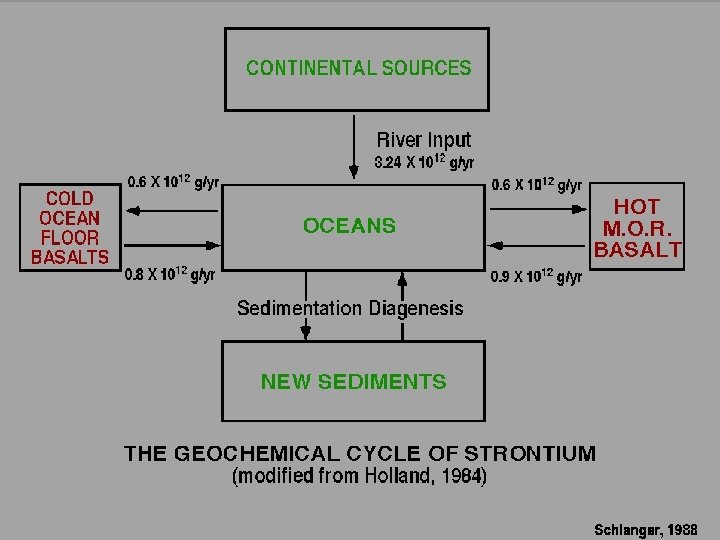
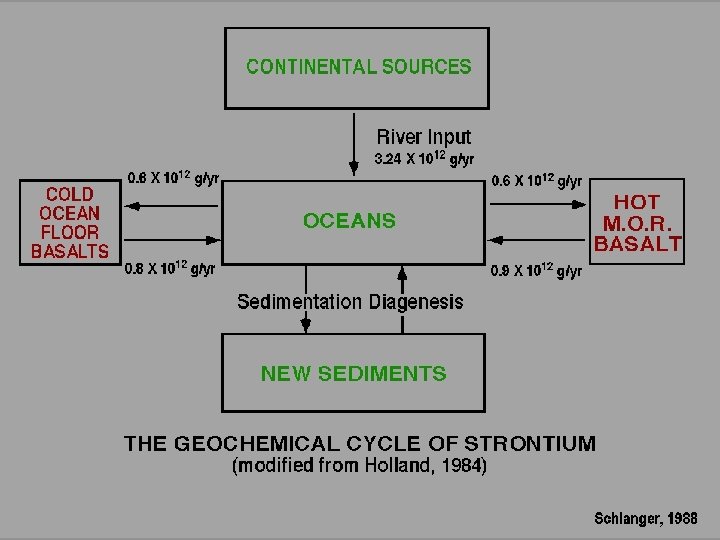
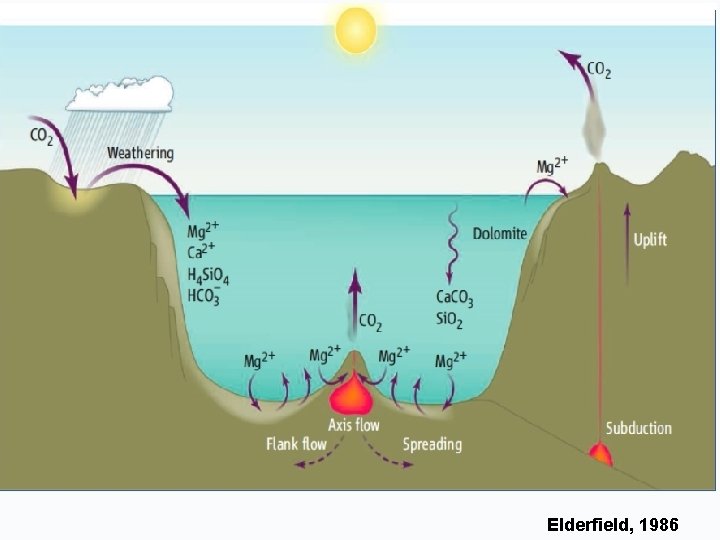
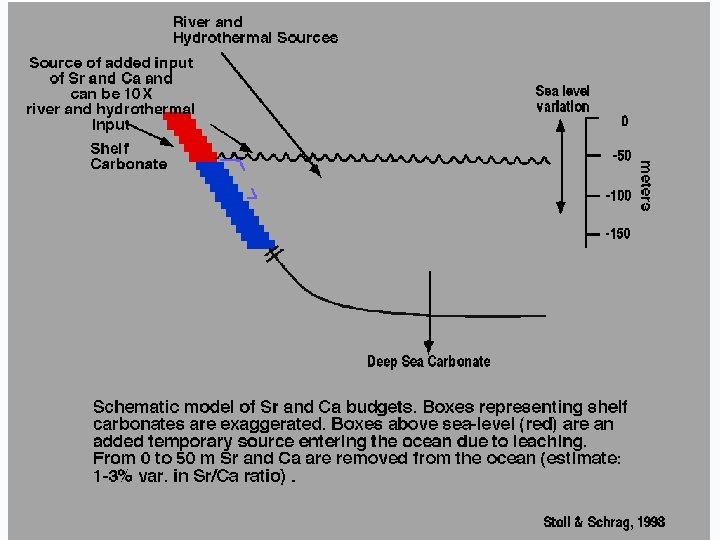
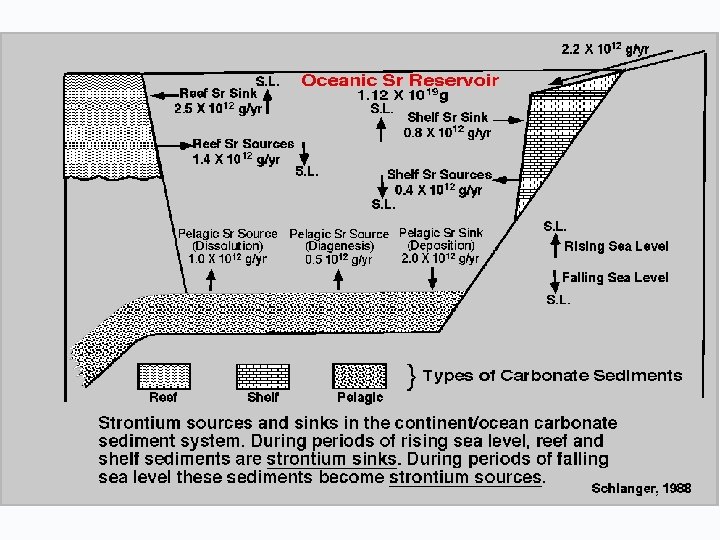
WHAT CONTROLS THE 87 Sr/86 Sr RATIO IN THE OCEANS?



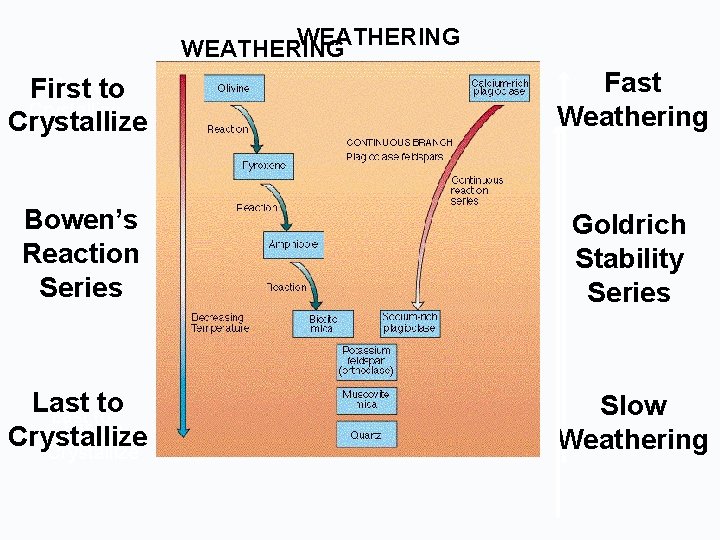
WEATHERING First toto First Crystallize Fast Weathering Bowen’s Reaction Series Goldrich Stability Series Last to Crystallize Slow Weathering






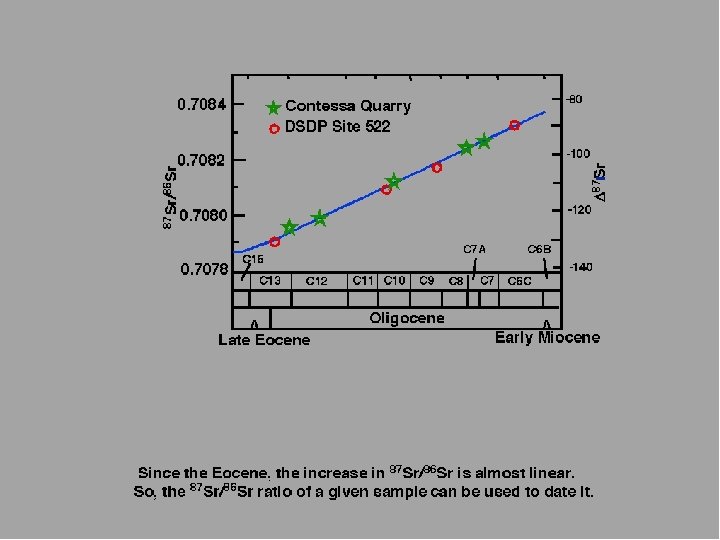
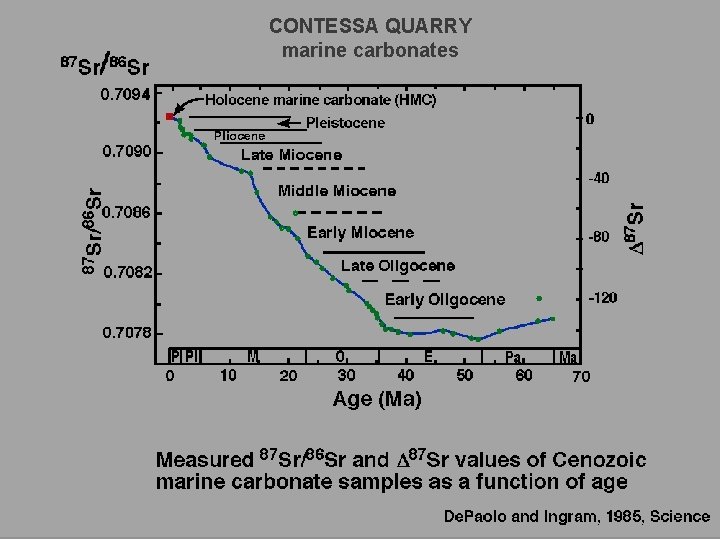
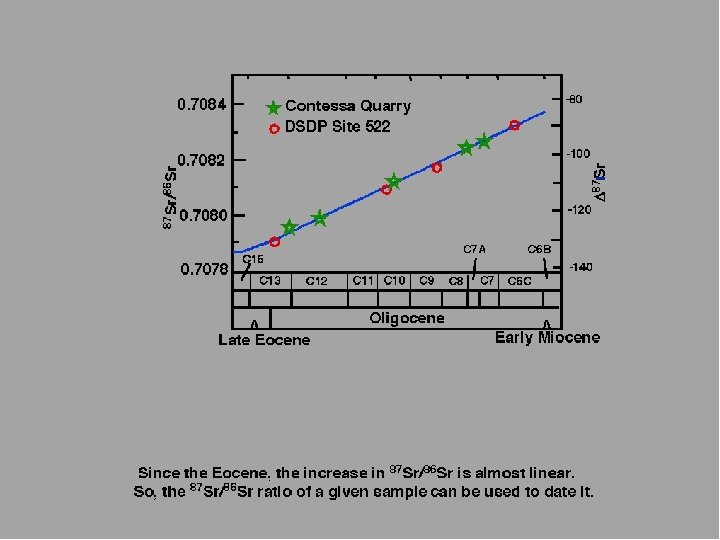
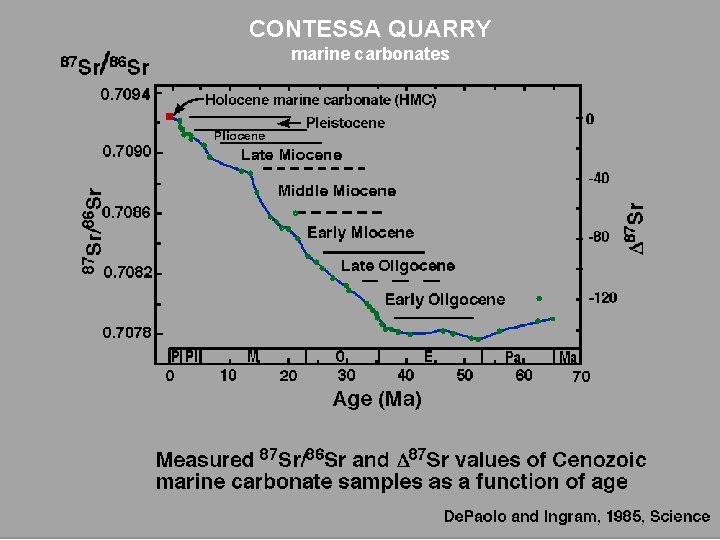
CONTESSA QUARRY marine carbonates

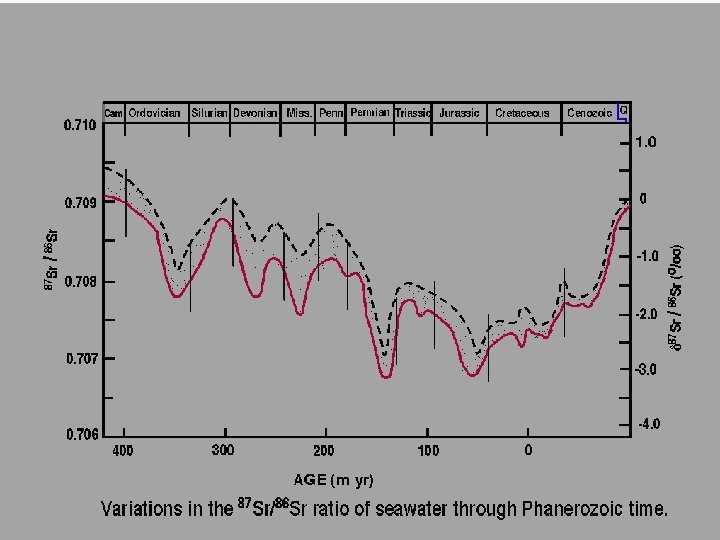
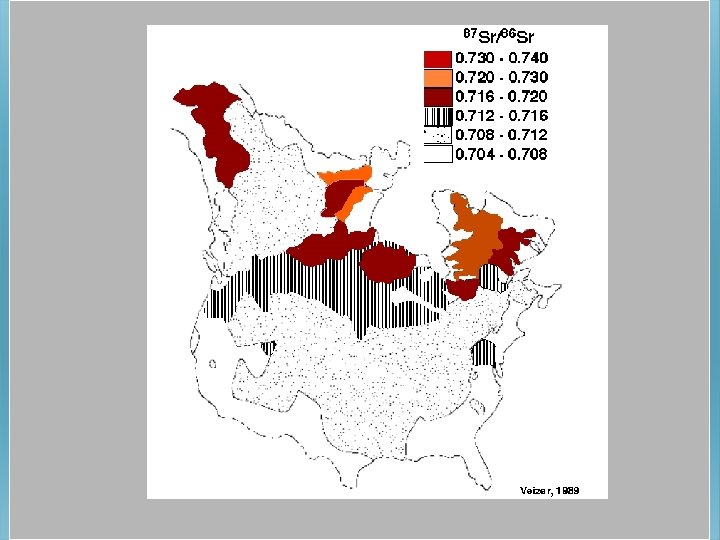
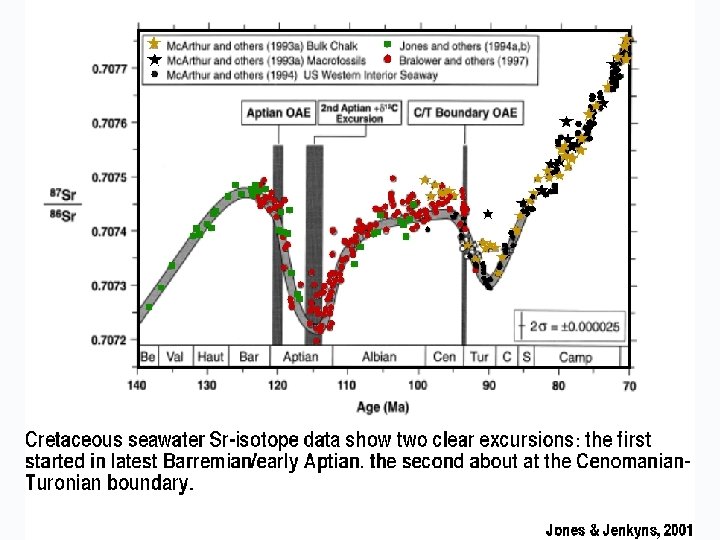
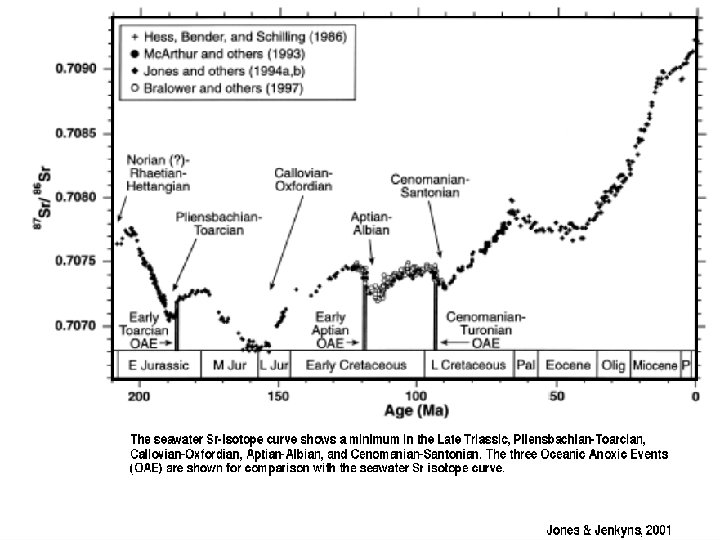
Modern seawater = 87 Sr/86 Sr = 0. 7092

CONTROLLED BY THE EXTENT THE Sr 2+ CAN SUBSTITUTE FOR Ca 2+ IN CALCIUM BEARING MINERALS

Two sources of 87 Sr in any material: (1) formed primordial nucleo-synthesis along with 84 Sr, 86 Sr and 88 Sr (2) radioactive decay of 87 Rb.

The ratio 87 Sr/86 Sr is the parameter typically reported in geologic investigations

WHAT CONTROLS THE 87 Sr/86 Sr RATIO IN THE OCEANS?



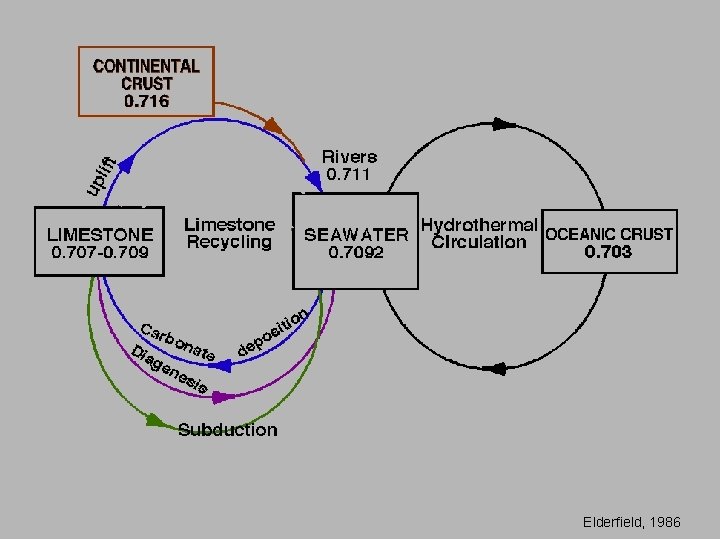
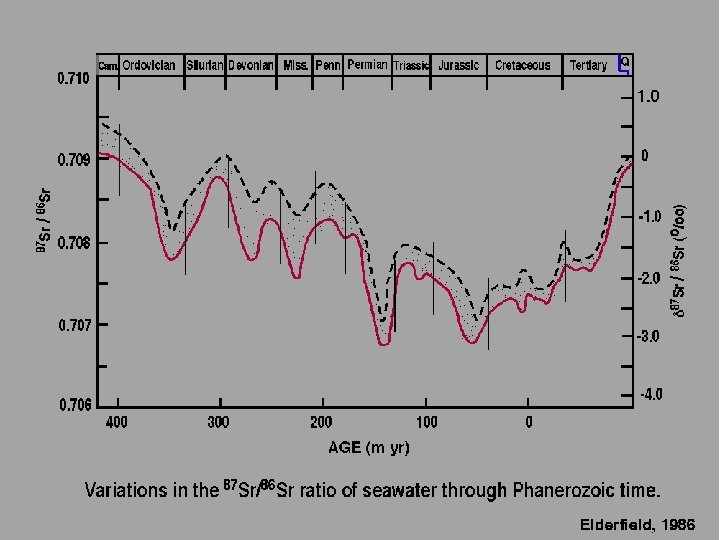
Elderfield, 1986

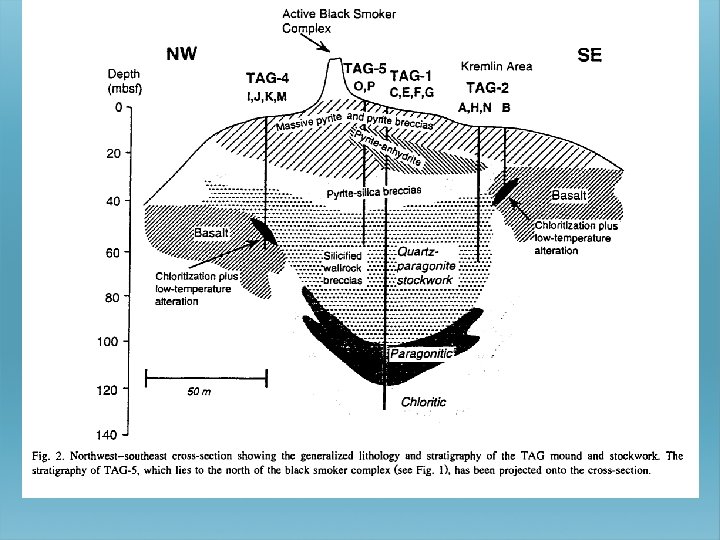
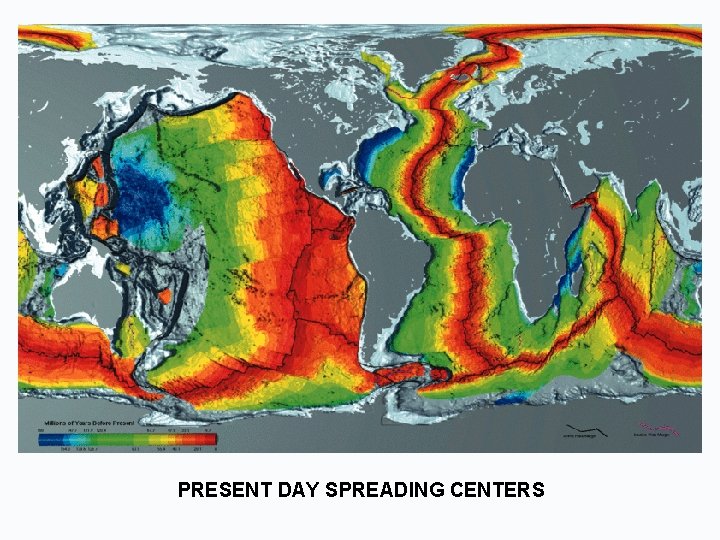
PRESENT DAY SPREADING CENTERS



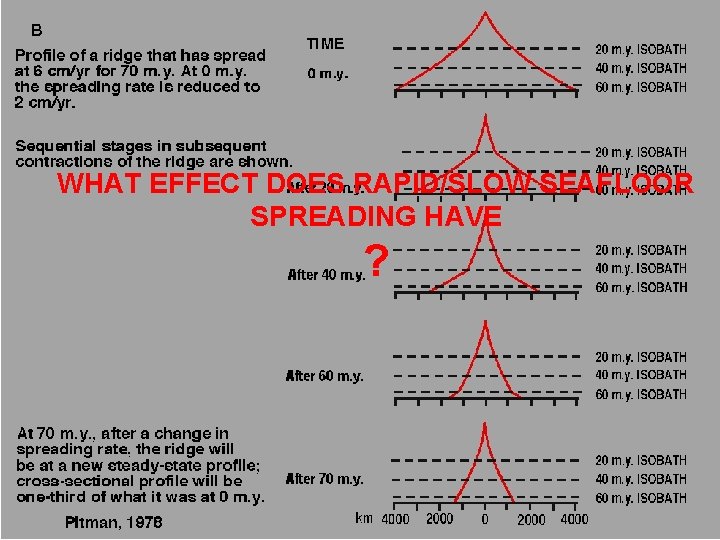
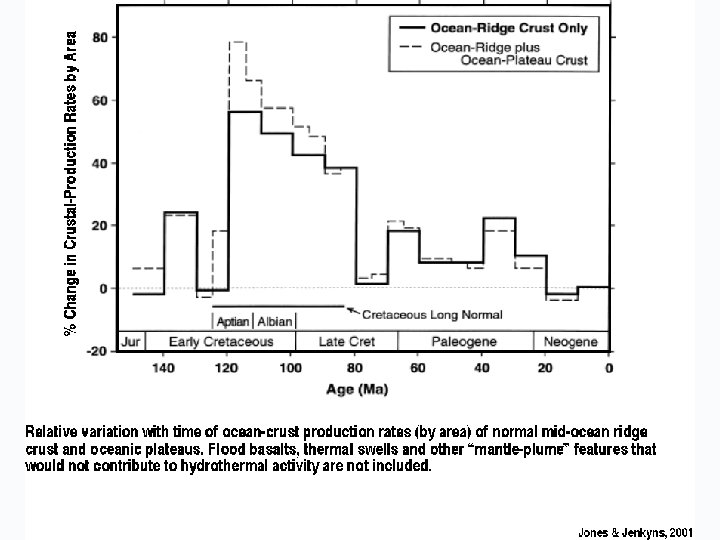
WHAT EFFECT DOES RAPID/SLOW SEAFLOOR SPREADING HAVE ?

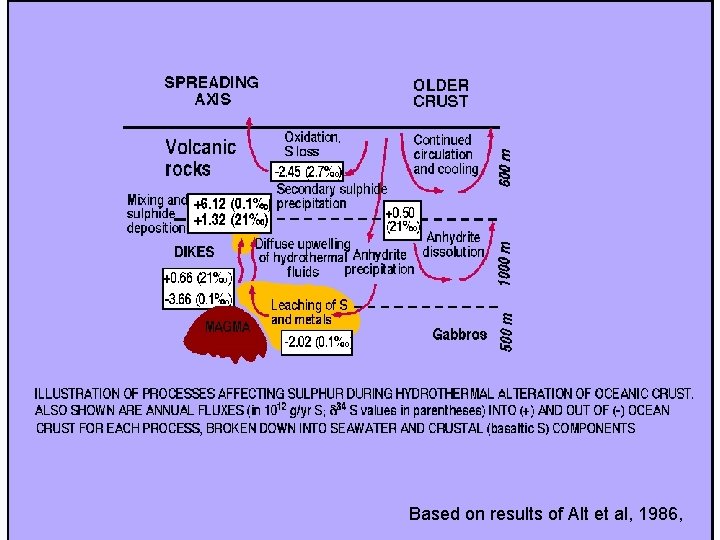
Based on results of Alt et al, 1986,

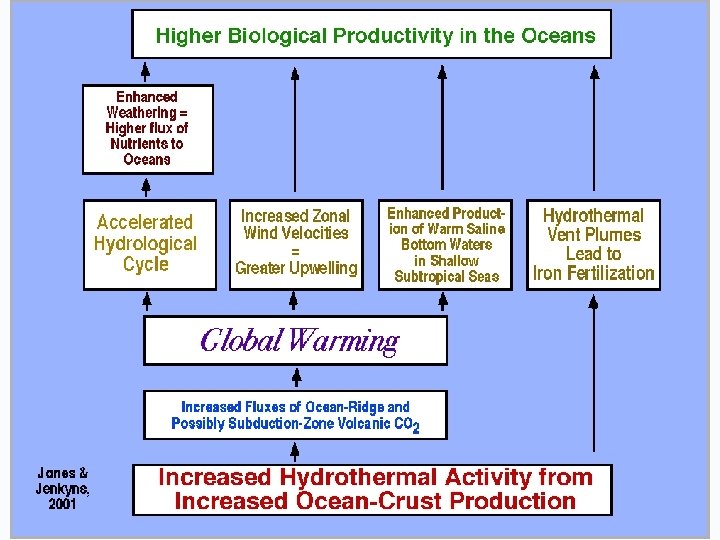
WHAT DOES RAPID SEA FLOOR SPREADING DO TO THE OCEANS?



Elderfield, 1986

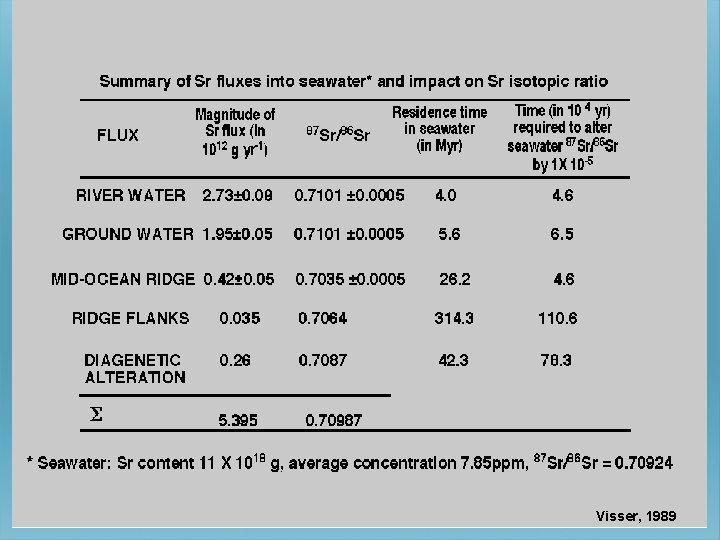
Visser, 1989





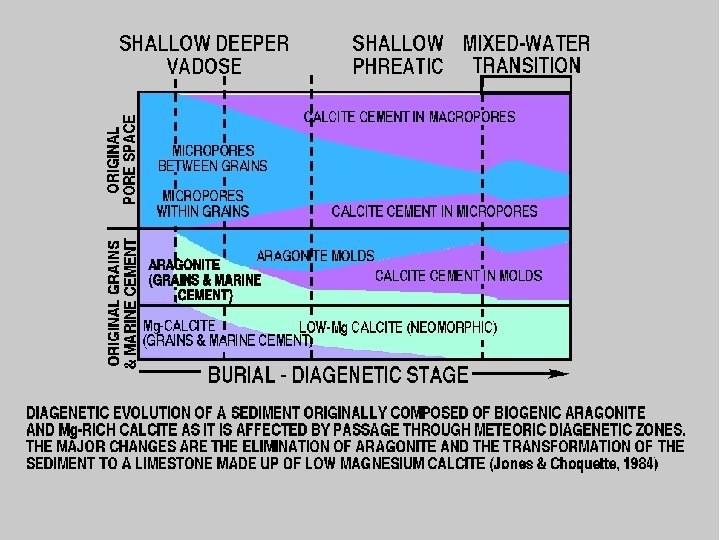
Sr IN GYPSUM REMAINS WITHIN SULPHATE EVEN WHEN IT DEHYDRATES BUT WHEN IT REHYDRATES, MUCH Sr IS LOST ALSO WHEN ARAGONITE GOES TO CALCITE MUCH Sr IS LOST






CONTESSA QUARRY marine carbonates


BACKGROUND During fractional crystallization, Sr tends to be come concentrated in the first minerals to crystallize, leaving Rb in the liquid phase. Hence, the Rb/Sr ratio in residual magma may increase over time, resulting in rocks with increasing Rb/Sr ratios with increasing differentiation. Highest ratios occur in pegmatites. Typically, Rb/Sr increases in the order plagioclase, hornblende, K-feldspar, biotite, muscovite. Therefore, given sufficient time for significant production (ingrowth) of radiogenic 87 Sr, measured 87 Sr/86 Sr values will be different in the minerals, increasing in the same order. The Rb-Sr dating method has been used extensively in dating rocks. If the initial amount of Sr is known or can be extrapolated, the age can be determined by measurement of the Rb and Sr concentrations and 87 Sr/86 Sr ratio. The dates indicate the true age of the minerals only if the rocks have not been subsequently altered.

Secondly, differences in the relative mobilities of water at scales ranging from inter-grain pores to the catchment scale may also profoundly affect 87 Sr/86 Sr (Bullen et al. , 1996). For example, the chemical composition and the resultant 87 Sr/86 Sr in immobile waters at a plagioclase-hornblende grain boundary versus a quartz-mica boundary will be different from eachother.

Third, a difference in the relative "effective" surface areas of minerals in one portion o f the rock unit will also cause differences in chemistry and isotopic composition; "poisoning" of reactive surfaces by organic coatings is an example of this kind of process. In a fundamental sense, because the waters in shallow systems are not in chemical equilibrium with the rocks, it is unrealistic to expect that waters along flowpaths within even a constant-mineralogy unit should have a constant 87 Sr/86 Sr. Instead, the waters moving along specific flowpaths slowly react with the rocks and gradually approach

The important concept for isotopic tracing is that Sr derived from any mineral through weathering reactions will have the same 87 Sr/86 Sr as the mineral itself. Therefore, differences in 87 Sr/86 Sr among ground waters require either (a) differences in mineralogy along contrasting flowpaths, or (b) differences in the relative amounts of Sr weathered from the same suite of minerals.
