Stroke Hyperglycemia Insulin Network Effort SHINE Trial An

















- Slides: 46

Stroke Hyperglycemia Insulin Network Effort (SHINE) Trial An Overview of SHINE NIH-NINDS U 01 NS 069498

Contents • • • Background Trial Overview Treatment Groups Outcomes I-SPOT Ancillary Study

SHINE Background • Most animal stroke studies show that hyperglycemia at stroke onset leads to worse outcomes than normoglycemia: – Larger strokes – More brain edema – Greater hemorrhagic stroke transformation J Cereb Blood Flow Metab 1997: 17: 553; Free Rad Biol Med 1997: 23: 986; Stroke 1989; 20: 646

SHINE Background • Most observational human studies reported independent associations between hyperglycemia (on admission as well as after hospitalization) and: – Greater infarct growth on MRI – Hemorrhagic stroke transformation when t. PA used – Worse functional outcomes Nat Rev Neurol 2010; 6: 145 Lancet Neurol 2012; 11: 261

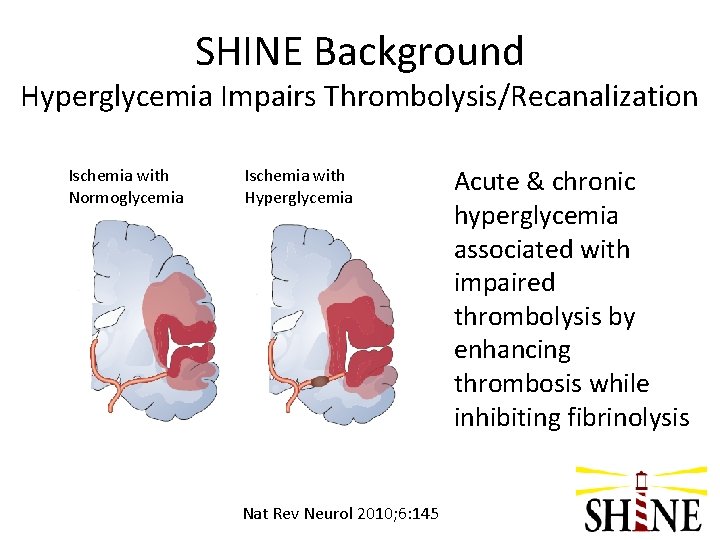
SHINE Background Hyperglycemia Impairs Thrombolysis/Recanalization Ischemia with Normoglycemia Ischemia with Hyperglycemia Nat Rev Neurol 2010; 6: 145 Acute & chronic hyperglycemia associated with impaired thrombolysis by enhancing thrombosis while inhibiting fibrinolysis

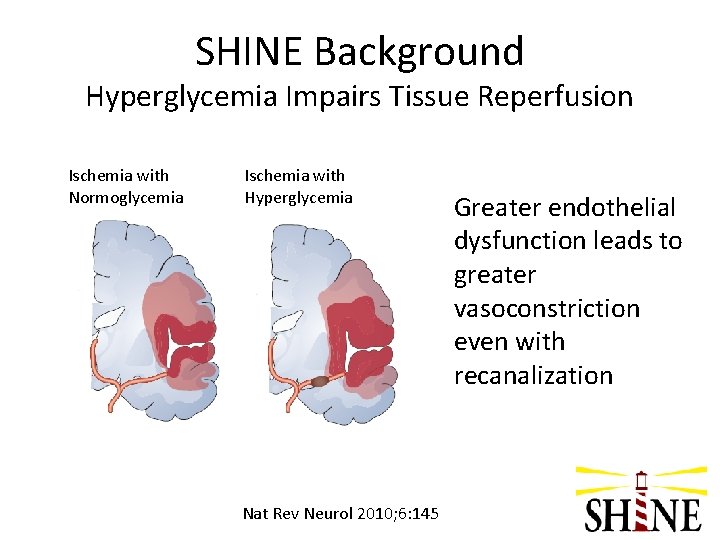
SHINE Background Hyperglycemia Impairs Tissue Reperfusion Ischemia with Normoglycemia Ischemia with Hyperglycemia Nat Rev Neurol 2010; 6: 145 Greater endothelial dysfunction leads to greater vasoconstriction even with recanalization

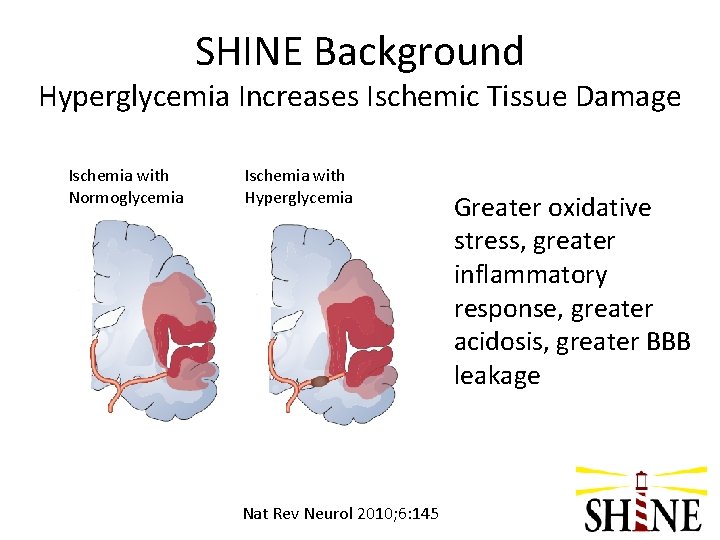
SHINE Background Hyperglycemia Increases Ischemic Tissue Damage Ischemia with Normoglycemia Ischemia with Hyperglycemia Nat Rev Neurol 2010; 6: 145 Greater oxidative stress, greater inflammatory response, greater acidosis, greater BBB leakage

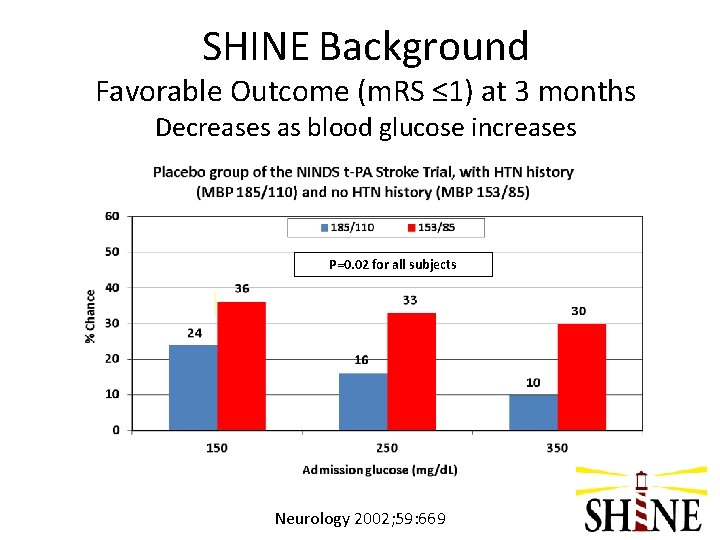
SHINE Background Favorable Outcome (m. RS ≤ 1) at 3 months Decreases as blood glucose increases P=0. 02 for all subjects Neurology 2002; 59: 669

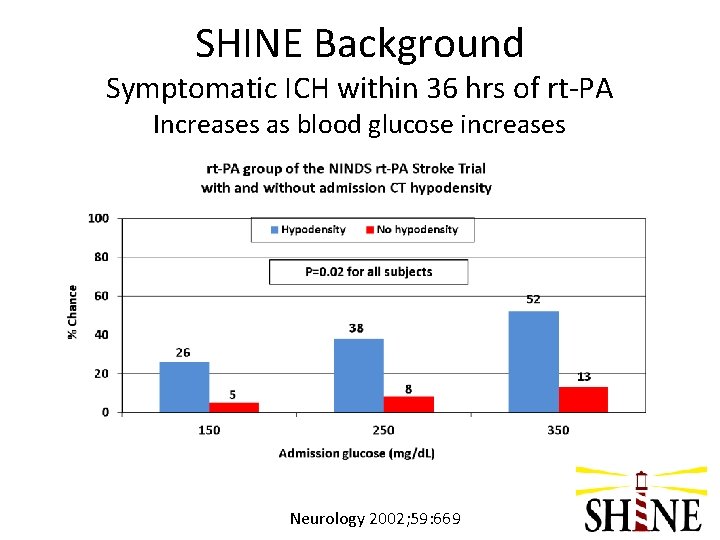
SHINE Background Symptomatic ICH within 36 hrs of rt-PA Increases as blood glucose increases Neurology 2002; 59: 669

SHINE Background Why might hyperglycemia during acute stroke be detrimental? Uncertain • • • Greater tissue lactic acidosis Increased local exitotoxic amino acids Impaired recovery of elevated intracellular Ca 2+ Impaired cerebral vasoreactivity Increased local free radicals Increased blood-brain barrier leakage Lancet Neurol 2012; 11: 261

SHINE Background • Uncertainty whether the observed clinical associations are cause or effect or associated without causation – Is it the hyperglycemia makes strokes worse leading to worse clinical outcomes or is it that more severe strokes and diabetes lead to worse clinical outcomes? • Uncertainty when hyperglycemia is harmful: – during acute stroke with or without reperfusion? – with lacunar and non-lacunar strokes?

SHINE Background Equipoise • • • Will acute correction of hyperglycemia improve functional outcomes? Will patients without DM benefit from acute hyperglycemia correction? What is the treatment time window? Is there a blood glucose threshold for favorable outcome? Will patients with lacunar strokes have better outcomes with higher glucose?

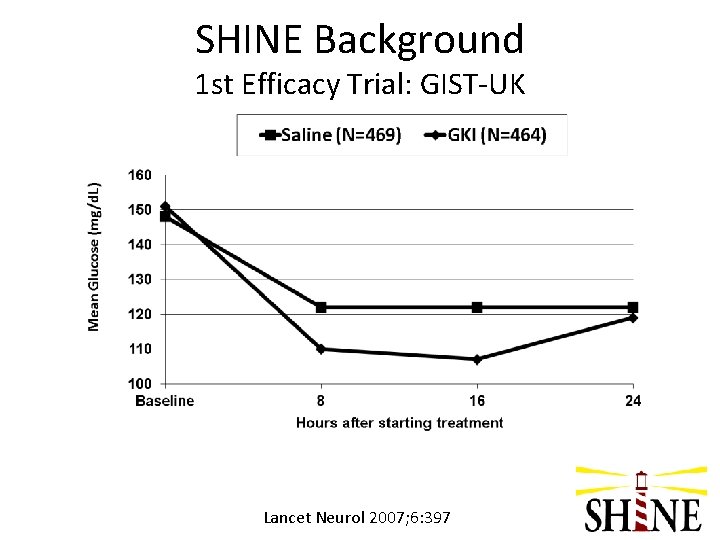
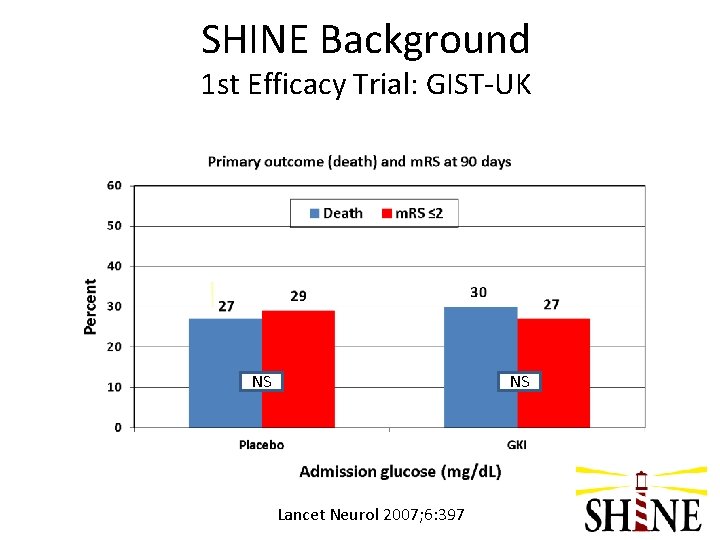
SHINE Background 1 st Efficacy Trial: Glucose Insulin in Stroke Trial – United Kingdom (GIST-UK) • • Stroke onset <24 hrs 83% patients without DM Relatively low blood glucose levels at baseline (~150 mg/d. L) Blood glucose in target range soon after treatment started in both groups Little separation of blood glucose levels between the 2 treatment groups (~10 mg/d. L) Trial stopped after only 40% of target enrolled; N=933 Primary outcome: death at 90 days Lancet Neurol 2007; 6: 397

SHINE Background 1 st Efficacy Trial: GIST-UK Lancet Neurol 2007; 6: 397

SHINE Background 1 st Efficacy Trial: GIST-UK NS NS Lancet Neurol 2007; 6: 397

SHINE Background Key observations from 2 pilot NINDS clinical trials • • • Hyperglycemia during acute stroke can be lowered in <6 hrs into target range with IV insulin protocols In patients without DM, hyperglycemia usually resolves rapidly without insulin treatment Hypoglycemia <60 mg/d. L occurs in up to 40% of patients, but is usually asymptomatic Hypoglycemia occurs less frequently with computerized decision support tools Phase III trial warranted Stroke 2008; 39: 384 -9, Stroke 2009; 40: 3804 -9

SHINE Trial NIH-NINDS U 01 5 NS 069498 • Multicenter (~60 sites), randomized, controlled trial • Phase III (definitive efficacy trial) • Predominantly hyperglycemic acute ischemic stroke patients • Comparison of standard SQ insulin vs IV insulin infusion • Funded by NIH-NINDS • Conducted in conjunction with NIH-NINDS funded Neurological Emergencies Treatment Trials Network (NETT) and NIH-NINDS Stroke. Net In J Stroke 2014; 9: 246 -51

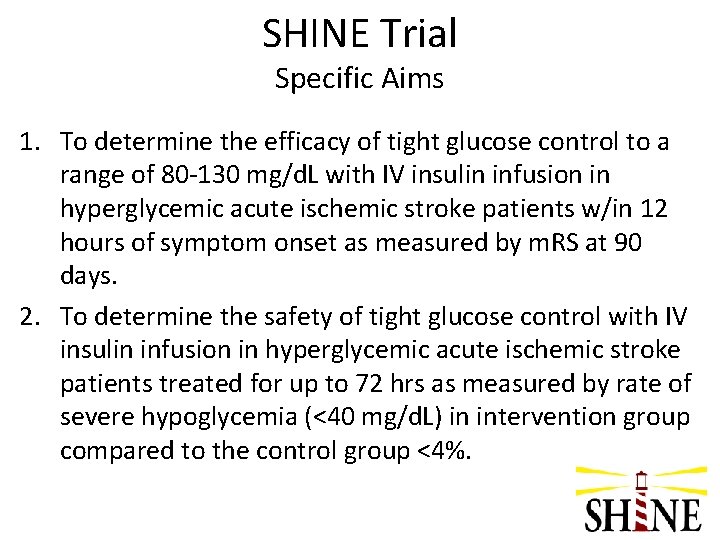
SHINE Trial Specific Aims 1. To determine the efficacy of tight glucose control to a range of 80 -130 mg/d. L with IV insulin infusion in hyperglycemic acute ischemic stroke patients w/in 12 hours of symptom onset as measured by m. RS at 90 days. 2. To determine the safety of tight glucose control with IV insulin infusion in hyperglycemic acute ischemic stroke patients treated for up to 72 hrs as measured by rate of severe hypoglycemia (<40 mg/d. L) in intervention group compared to the control group <4%.

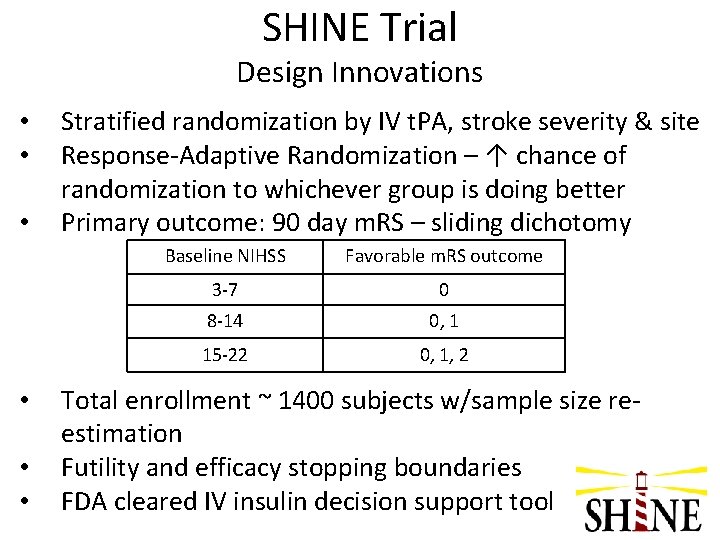
SHINE Trial Design Innovations • • • Stratified randomization by IV t. PA, stroke severity & site Response-Adaptive Randomization – ↑ chance of randomization to whichever group is doing better Primary outcome: 90 day m. RS – sliding dichotomy Baseline NIHSS Favorable m. RS outcome 3 -7 0 8 -14 0, 1 15 -22 0, 1, 2 Total enrollment ~ 1400 subjects w/sample size reestimation Futility and efficacy stopping boundaries FDA cleared IV insulin decision support tool

SHINE Trial ® Decision Support Tool - Gluco. Stabilizer • Designed at Indiana University/Clarian Health • Licensed by Medical Decision Network • FDA cleared and commercially available – used in >65, 000 patients • Considers individual patient response to insulin • Severe hypoglycemia rate (<40 mg/d. L) - 1. 67% of patients and 0. 07% of all glucose checks • Adherence to the recommendations – 99% – 91 -98% in literature for decision support tools – 97% in GRASP Crit Care Med 2008; 36: 1787 -95, Stroke 2009; 40: 3804 -9

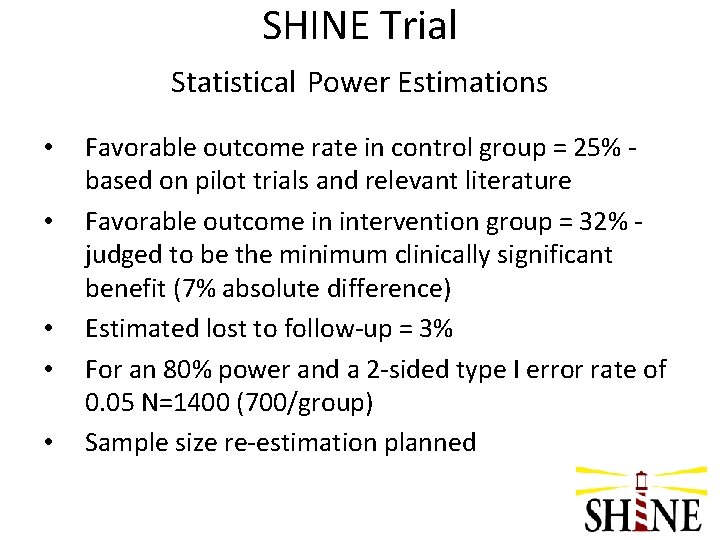
SHINE Trial Statistical Power Estimations • • • Favorable outcome rate in control group = 25% based on pilot trials and relevant literature Favorable outcome in intervention group = 32% judged to be the minimum clinically significant benefit (7% absolute difference) Estimated lost to follow-up = 3% For an 80% power and a 2 -sided type I error rate of 0. 05 N=1400 (700/group) Sample size re-estimation planned

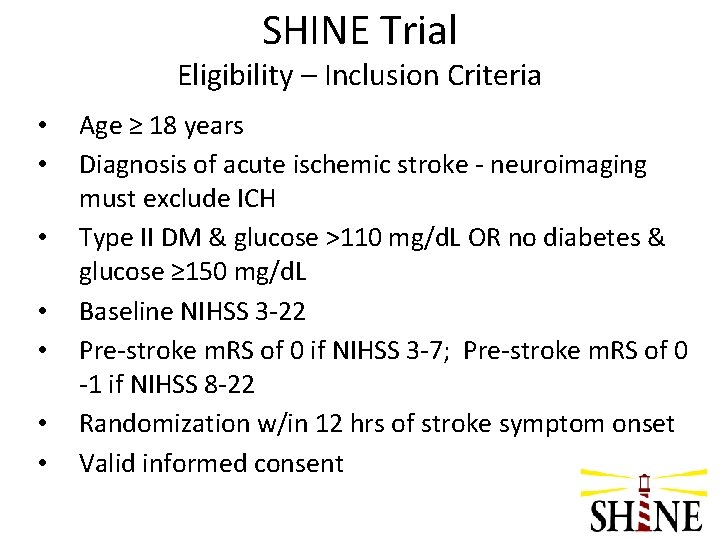
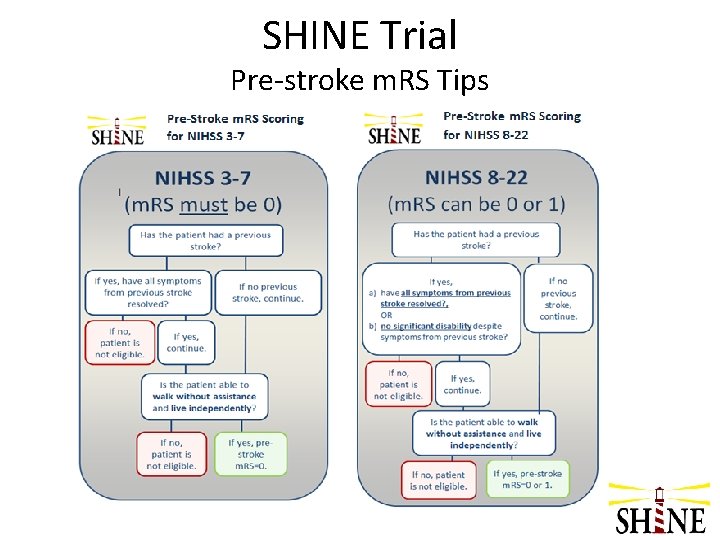
SHINE Trial Eligibility – Inclusion Criteria • • Age ≥ 18 years Diagnosis of acute ischemic stroke - neuroimaging must exclude ICH Type II DM & glucose >110 mg/d. L OR no diabetes & glucose ≥ 150 mg/d. L Baseline NIHSS 3 -22 Pre-stroke m. RS of 0 if NIHSS 3 -7; Pre-stroke m. RS of 0 -1 if NIHSS 8 -22 Randomization w/in 12 hrs of stroke symptom onset Valid informed consent

SHINE Trial Eligibility - Exclusion Criteria • Type I DM • Condition confounding baseline neurological exam • Neurological or psychiatric illness likely to confound primary outcome assessment at 90 days • Any experimental therapy for enrollment stroke – standard care IV t. PA within 4. 5 hrs, IA t. PA & IA therapies w/ FDA cleared devices allowed. Non FDA cleared devices excluded • • Inability to follow the protocol or return for 90 day f/u Serious conditions with unlikely 90 day survival Pregnancy or breast-feeding Any renal dialysis

SHINE Trial Pre-stroke m. RS Tips

SHINE Trial Baseline NIHSS & SHINE Eligibility • • • NIHSS must be 3 -22 and assessed ≤ 30 minutes prior to randomization Prior stroke deficits must not confound the acute stroke deficits Intubated patients are “untestable” for dysarthria and could be enrolled only if the NIHSS was scored before intubation and ≤ 30 minutes prior to randomization

SHINE Trial Qualifying Blood Glucose & SHINE Eligibility • • • Must be done at the enrolling hospital Must be a finger stick (capillary) blood Consider rechecking if close to qualifying level and time remains within the 12 hr window

SHINE Trial Blinding Strategy • • • During acute treatment, single blind for patients/family All subjects receive study IV infusions and study SQ injections – some contain insulin & some contain saline placebo – only the clinical team members know which solutions contain insulin The primary & secondary outcomes are assessed by a blinded investigator resulting in a double blind outcome assessment

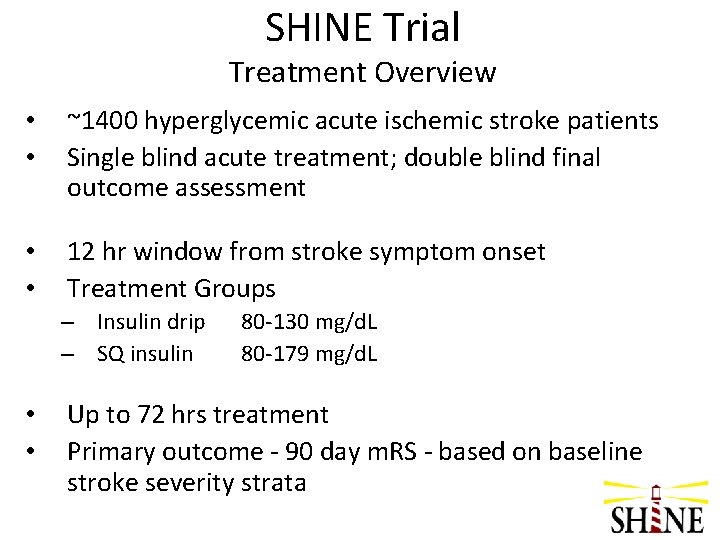
SHINE Trial Treatment Overview • • ~1400 hyperglycemic acute ischemic stroke patients Single blind acute treatment; double blind final outcome assessment • • 12 hr window from stroke symptom onset Treatment Groups – Insulin drip – SQ insulin • • 80 -130 mg/d. L 80 -179 mg/d. L Up to 72 hrs treatment Primary outcome - 90 day m. RS - based on baseline stroke severity strata

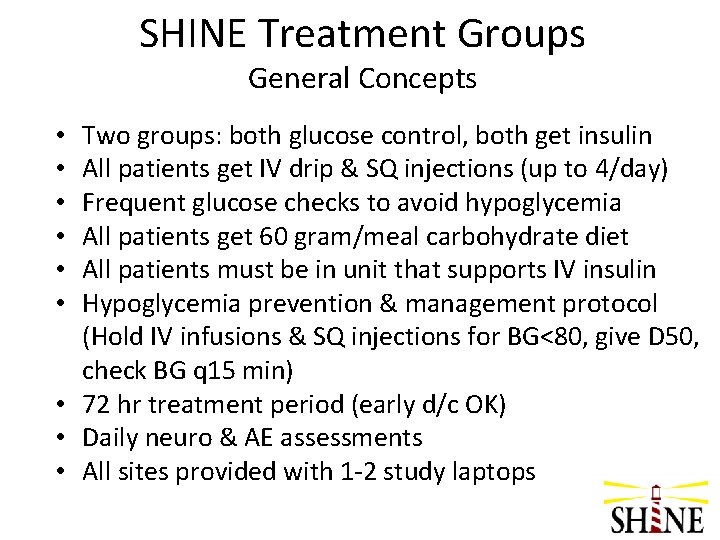
SHINE Treatment Groups General Concepts Two groups: both glucose control, both get insulin All patients get IV drip & SQ injections (up to 4/day) Frequent glucose checks to avoid hypoglycemia All patients get 60 gram/meal carbohydrate diet All patients must be in unit that supports IV insulin Hypoglycemia prevention & management protocol (Hold IV infusions & SQ injections for BG<80, give D 50, check BG q 15 min) • 72 hr treatment period (early d/c OK) • Daily neuro & AE assessments • All sites provided with 1 -2 study laptops • • •

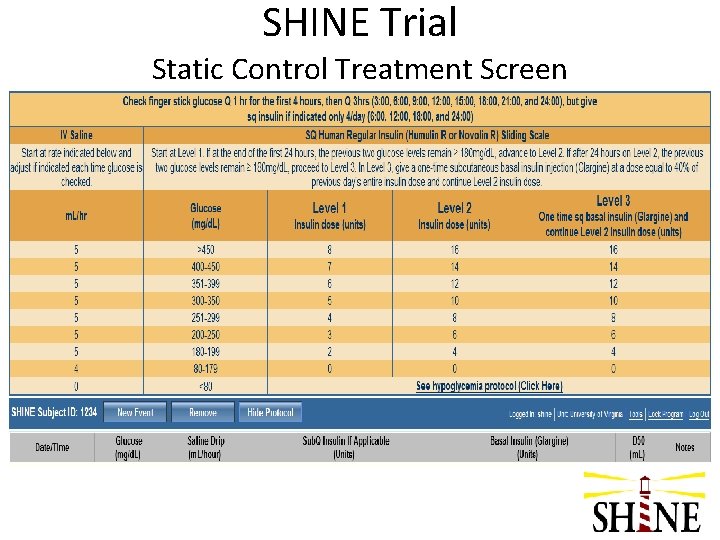
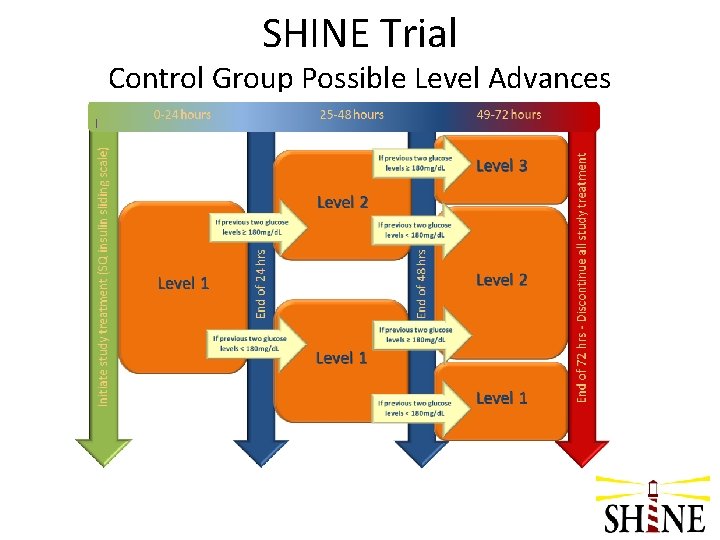
SHINE Trial Control Group Target glucose: 80 -179 mg/d. L Glucose checks: q 1 -q 3 hrs (+/- 15 min) IV infusion: normal saline (placebo) – 4 -5 cc/hr SQ injections: human regular insulin per sliding scale, given only @ 6: 00, 12: 00, 18: 00, & 24: 00 • Escalating doses of insulin per sliding scale if not in target, including a one-time dose of basal insulin @ 48 hrs in level 3 • Laptop displays SQ insulin sliding scale dosing and hypoglycemia protocol • •

SHINE Trial Static Control Treatment Screen

SHINE Trial Control Group Possible Level Advances

SHINE Trial Intervention Group • Target glucose: 80 -130 mg/d. L • Glucose checks: q 1 -2 hrs (+/- 15 min) per Gluco. Stabilizer® • IV infusion: human regular insulin – rate directed by Gluco. Stabilizer® program based on glucose levels • SQ injections: – Normal saline (placebo) 0. 05 cc @ 9: 00 & 21: 00 if NPO or on continuous tube feeds OR – Rapid acting analog insulin after meals

SHINE Trial Outcomes • • Primary efficacy: baseline stroke severity adjusted 90 day m. RS (sliding dichotomy) Secondary efficacy: – 90 day NIHSS – 90 day Barthel Index – 90 day SSQOL (Stroke Specific Quality of Life) • Primary safety: severe hypoglycemia (BG<40 mg/d. L)

SHINE Trial Centers and Leadership • • • Karen C. Johnston, MD, MSc (UVA) – Administrative PI Askiel Bruno, MD, MS (GRU) – Protocol PI Christiana Hall, MD, MS (UTSW) – Recruitment PI William Barsan, MD (Univ of Michigan) NETT Clinical Coordinating Center PI Valerie Durkalski, Ph. D (MUSC) NETT Statistics and Data Management Center PI Clinical enrolling sites: – – – NETT network – 22 hub/spoke complexes Stroke. Net sites to be included 11 ancillary sites

SHINE Trial Centers

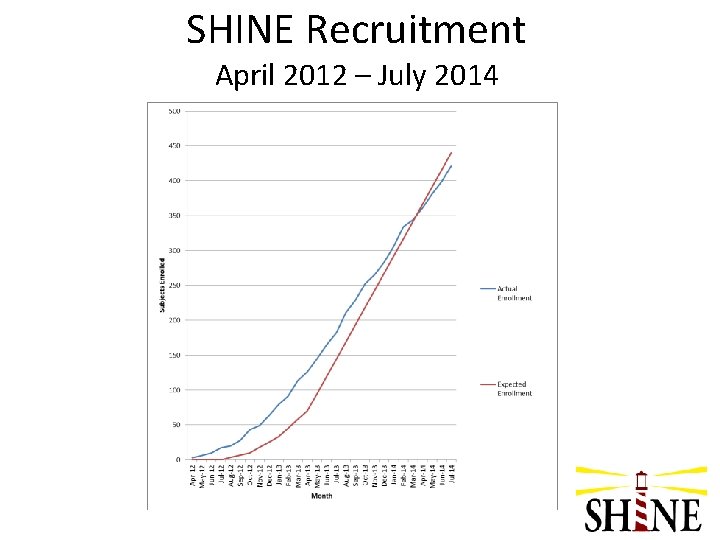
SHINE Recruitment April 2012 – July 2014

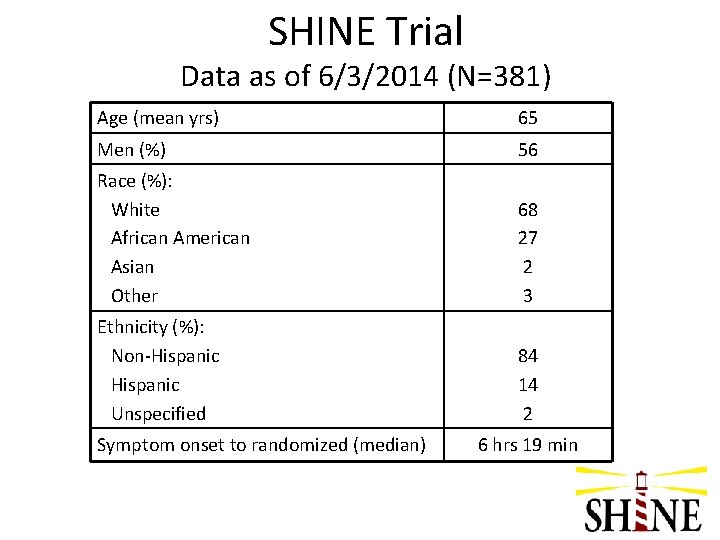
SHINE Trial Data as of 6/3/2014 (N=381) Age (mean yrs) 65 Men (%) 56 Race (%): White African American Asian Other 68 27 2 3 Ethnicity (%): Non-Hispanic Unspecified 84 14 2 Symptom onset to randomized (median) 6 hrs 19 min

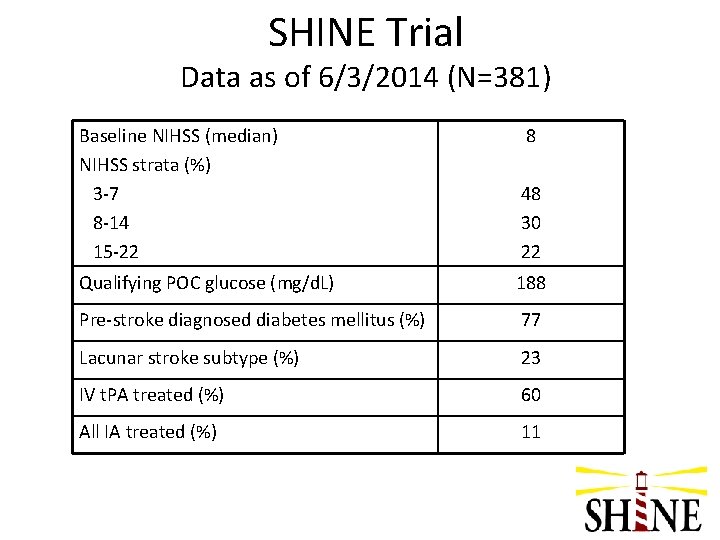
SHINE Trial Data as of 6/3/2014 (N=381) Baseline NIHSS (median) NIHSS strata (%) 3 -7 8 -14 15 -22 8 48 30 22 Qualifying POC glucose (mg/d. L) 188 Pre-stroke diagnosed diabetes mellitus (%) 77 Lacunar stroke subtype (%) 23 IV t. PA treated (%) 60 All IA treated (%) 11

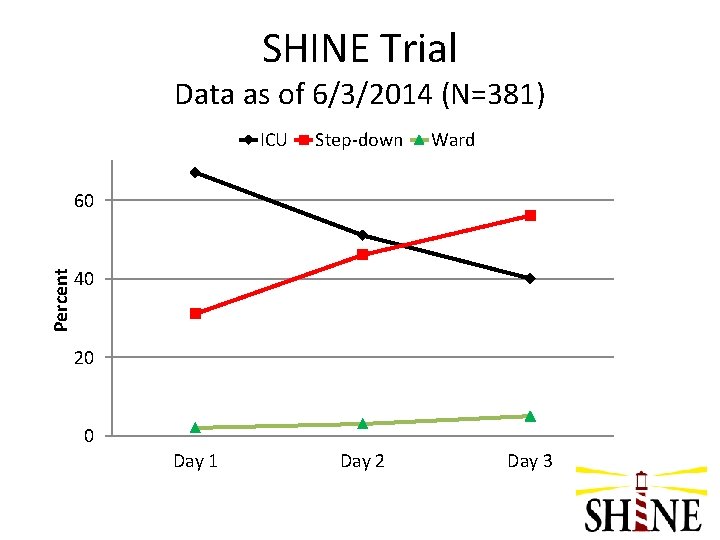
SHINE Trial Data as of 6/3/2014 (N=381) ICU Step-down Ward Percent 60 40 20 0 Day 1 Day 2 Day 3

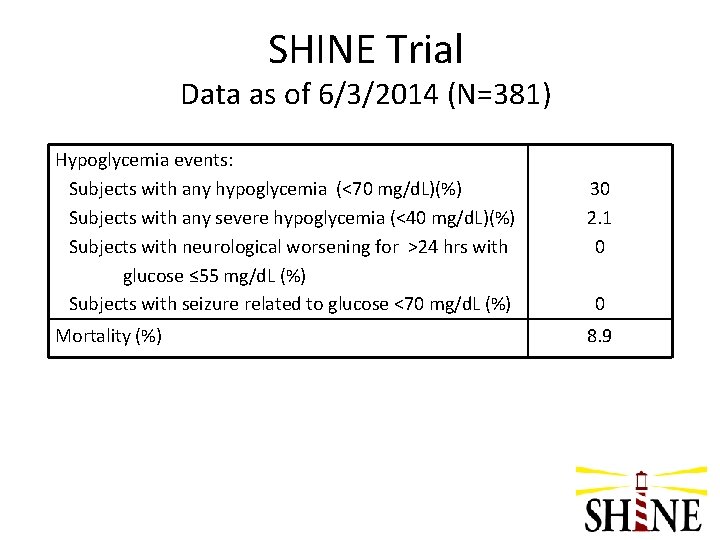
SHINE Trial Data as of 6/3/2014 (N=381) Hypoglycemia events: Subjects with any hypoglycemia (<70 mg/d. L)(%) Subjects with any severe hypoglycemia (<40 mg/d. L)(%) Subjects with neurological worsening for >24 hrs with glucose ≤ 55 mg/d. L (%) Subjects with seizure related to glucose <70 mg/d. L (%) Mortality (%) 30 2. 1 0 0 8. 9

I-SPOT & SHINE Insights on Selected Procoagulation Markers and Outcomes in Stroke Trial - 1 U 01 NS 079077 • I-SPOT seeks to determine if intense BG control compared with standard BG treatment results in reductions in markers of blood coagulation • Determine the relationships between blood glucose, markers of blood coagulation and thrombosis, and SHINE clinical outcomes • Markers: – – – Whole blood Tissue Factor - PCA Coagulation factors VII, VIIa, and VIII Plasma TAT, Fragmin 1. 2, D-dimer; Tissue factor pathway inhibitor Plasminogen activator inhibitor-1

I-SPOT & SHINE Insights on Selected Procoagulation Markers and Outcomes in Stroke Trial – 1 U 01 NS 079077 • I-SPOT is a multi-center study nested within the SHINE trial • A subset of 315 patients enrolled in the SHINE trial will be enrolled in the I-SPOT study • Baseline & 48 -hr blood samples collected, spun, frozen, & batch sent to the main lab at Temple University

I-SPOT & SHINE Insights on Selected Procoagulation Markers and Outcomes in Stroke Trial – 1 U 01 NS 079077 Inclusion: • Must also be enrolled in the SHINE trial Exclusions: • Thrombolytic treatments, e. g. IV or IA t. PA • Full anticoagulation, e. g. heparin, warfarin, etc. • Moderate-severe hepatic insufficiency (INR>1. 5, if known, or history of variceal bleeding, or hepatic encephalopathy) • History of thrombotic or hypercoagulable condition: e. g. antiphospholipid antibody syndrome, antithrombin III, Protein C or S deficiencies, congenital/Inherited factor deficiencies, sickle cell disease.

I-SPOT & SHINE Insights on Selected Procoagulation Markers and Outcomes in Stroke Trial – 1 U 01 NS 079077 • Allowed interventions – SQ DVT prophylactic anticoagulation doses – Standard antiplatelet treatments
