Stress Response Pathway Ensemble A New Paradigm in

Stress Response Pathway Ensemble: A New Paradigm in High Throughput Toxicity Screening Steve Simmons US EPA Office of Research and Development RTP, NC The Mc. Kim Conferences September 2008 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch

Outline • The Challenge Before Us • Stress Response Pathways as Toxicity Pathways • Stress Response Assays • Implementation to HTS • What does this have to with QSAR? Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch

Problem/Challenge • Large number of environmental compounds that currently need characterization and prioritization for further screening • Limited testing resources • Current approaches are slow, laborious, and expensive • Ethical need to reduce animal use in toxicity testing • In vitro –omics approaches too expensive for screening applications and data interpretation problematic • Multiple alternative approaches needed, assay cost to be minimized 2 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
Solution • Rapid, inexpensive, reproducible and predictive assays that allow for effective screening of large numbers of compounds to enable prioritization for further characterization • In vitro assays amenable to high-throughput screening, preferably using human cells and tissues that are significantly more economical and practical than –omic methods and traditional in vivo testing methods 3 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
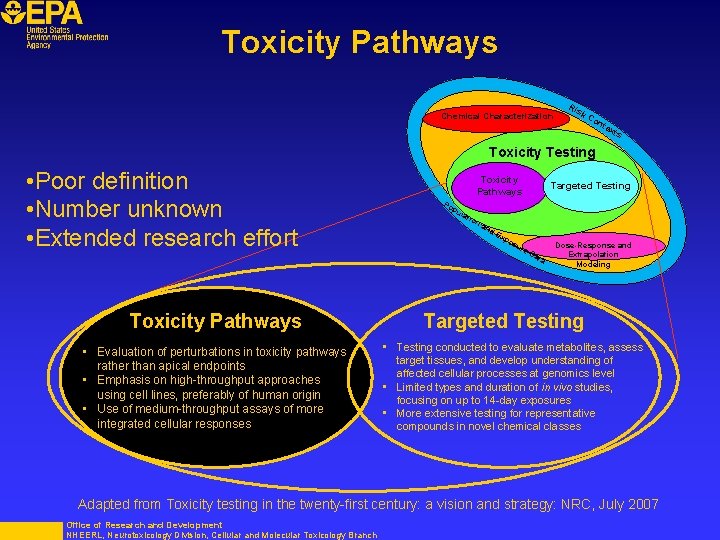
Toxicity Pathways Chemical Characterization Ris k. C on tex ts Toxicity Testing • Poor definition • Number unknown • Extended research effort Toxicity Pathways • Evaluation of perturbations in toxicity pathways rather than apical endpoints • Emphasis on high-throughput approaches using cell lines, preferably of human origin • Use of medium-throughput assays of more integrated cellular responses Toxicity Pathways Po p ula tio na nd Ex po su re Targeted Testing Da ta Dose-Response and Extrapolation Modeling Targeted Testing • Testing conducted to evaluate metabolites, assess target tissues, and develop understanding of affected cellular processes at genomics level • Limited types and duration of in vivo studies, focusing on up to 14 -day exposures • More extensive testing for representative compounds in novel chemical classes Adapted from Toxicity testing in the twenty-first century: a vision and strategy: NRC, July 2007 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch

Toxicity Pathways • Are all cellular pathways potential toxicity pathways? • If so, are all pathways created equal, or are some more important than others? • How do you determine importance/priority? q Cover the most chemical space? q Easiest to model? Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 5

Stress Responses Sense Perturbations Exposure Tissue Dose Adapted from: Toxicity Testing in the Twenty-first Century: A Vision and a Strategy, National Research Council. 2007. Biologic Interaction Perturbation Normal Biologic Inputs Biologic Function Reversible Adaptive Stress Response Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch Early Cellular Changes Cell Injury Irreversible 6 Morbidity and Mortalilty

Adaptive Stress-Response Pathways • Protective signaling pathways activated in response to environmental insults such as chemical toxicity • Present in all cells and highly conserved • Broad indicators of cellular toxicity • Triggered at low doses before more apical effects such as cell death or apoptosis • A few (<10) key cellular stress pathways identified • Pathways well-characterized and classified mechanistically mode of action info? ? 7 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch

Adaptive Stress Response Pathways Oxidative stress DNA damage Heat shock ER stress Hypoxia/Anoxia Inflammation Heavy metal stress Osmotic stress* 8 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
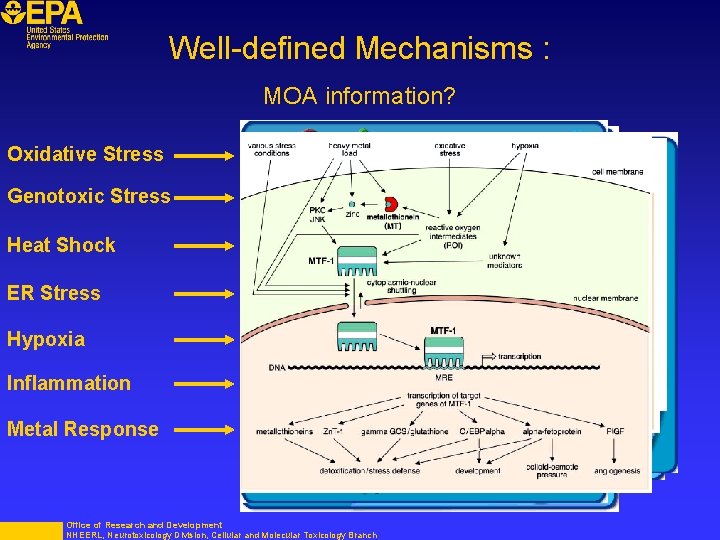
Well-defined Mechanisms : MOA information? Oxidative Stress Genotoxic Stress Heat Shock ER Stress Hypoxia Inflammation Metal Response Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
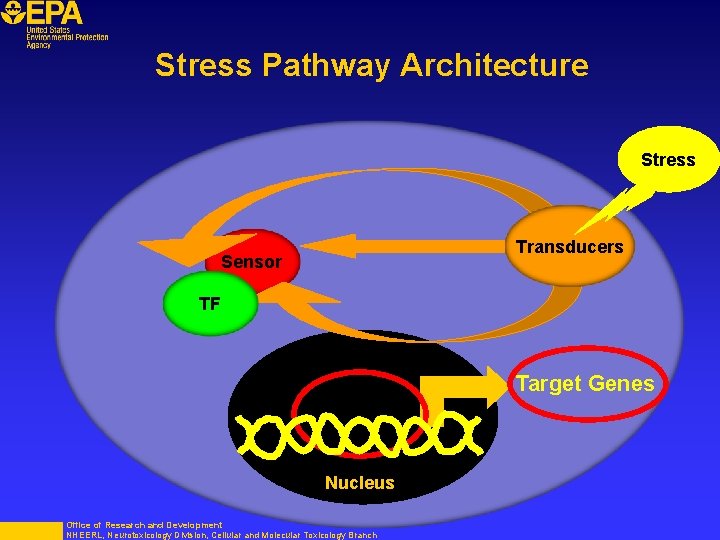
Stress Pathway Architecture Stress Transducers Sensor TF Target Genes Nucleus Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
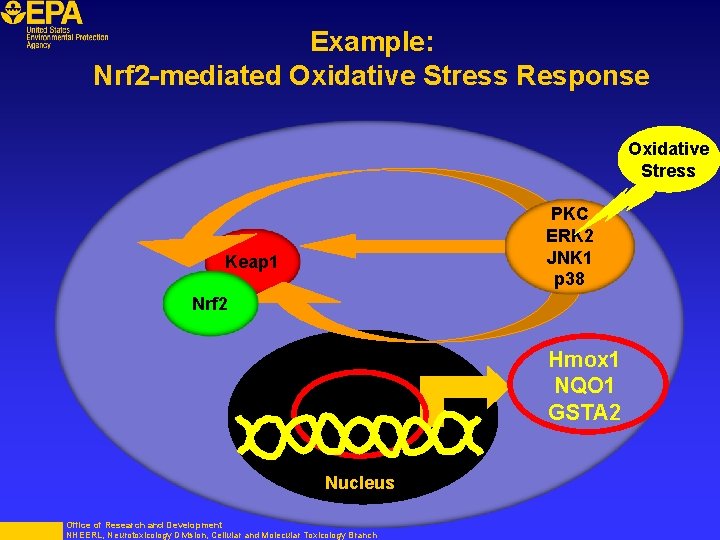
Example: Nrf 2 -mediated Oxidative Stress Response Oxidative Stress PKC ERK 2 JNK 1 p 38 Keap 1 Nrf 2 Hmox 1 NQO 1 GSTA 2 Nucleus Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
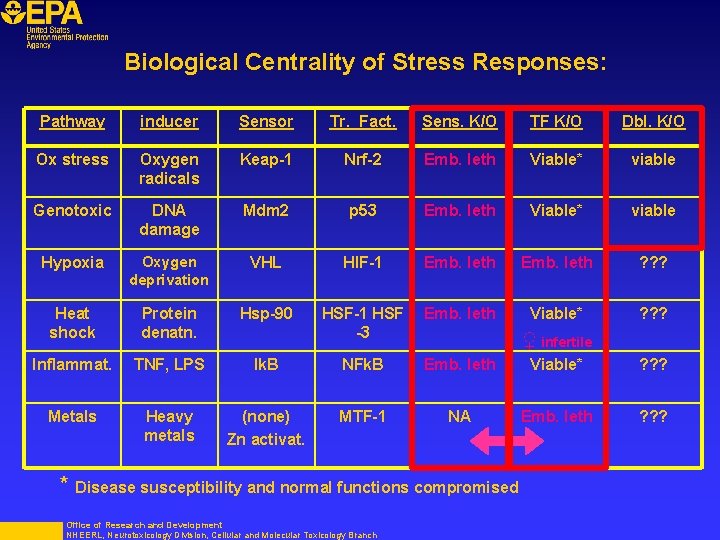
Biological Centrality of Stress Responses: Pathway inducer Sensor Tr. Fact. Sens. K/O TF K/O Dbl. K/O Ox stress Oxygen radicals Keap-1 Nrf-2 Emb. leth Viable* viable Genotoxic DNA damage Mdm 2 p 53 Emb. leth Viable* viable Hypoxia Oxygen deprivation VHL HIF-1 Emb. leth ? ? ? Heat shock Protein denatn. Hsp-90 HSF-1 HSF -3 Emb. leth Viable* ? ? ? Inflammat. TNF, LPS Ik. B NFk. B Emb. leth Viable* ? ? ? Metals Heavy metals (none) Zn activat. MTF-1 NA Emb. leth ? ? ? ♀ infertile * Disease susceptibility and normal functions compromised Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch

Stress-Response Pathways 1. Stress pathways share a common pattern of organization 2. Stimulation of a stress pathway results in activated transcription factor 3. The transcription factor serve as a nodal point for multiple “toxicity pathways” 4. Activated transcription factor up-regulates unique target genes Regulatory elements of target genes can be used to measure pathway activation! Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
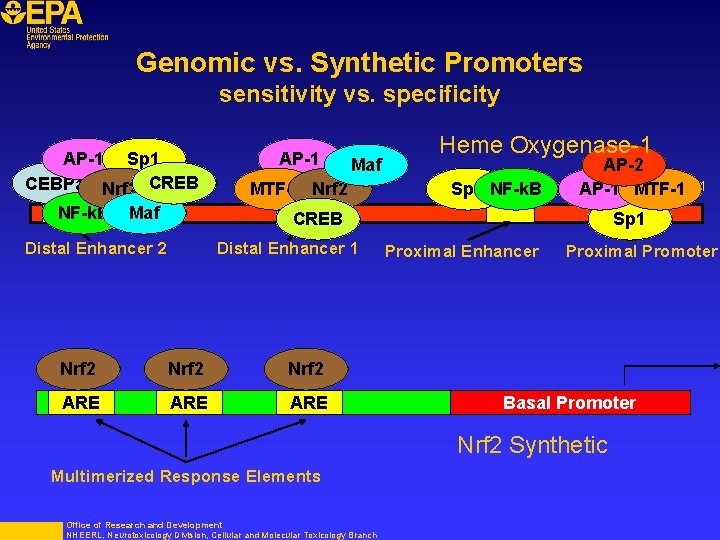
Genomic vs. Synthetic Promoters sensitivity vs. specificity AP-1 Sp 1 -12 kb CEBPa Nrf 2 CREB NF-k. B Maf AP-1 Maf MTF-1 Nrf 2 Heme Oxygenase-1 Sp 1 NF-k. B AP-2 AP-1 MTF-1 +1 CREB Distal Enhancer 2 Distal Enhancer 1 Nrf 2 ARE ARE Sp 1 Proximal Enhancer Proximal Promoter Basal Promoter Nrf 2 Synthetic Multimerized Response Elements Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
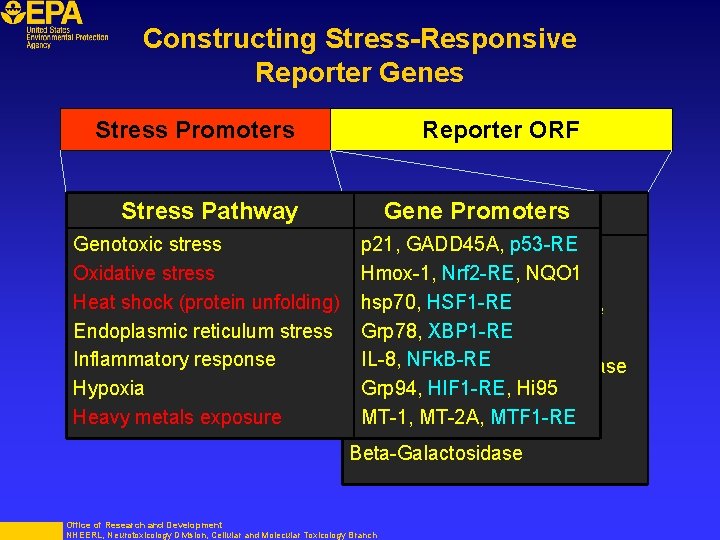
Constructing Stress-Responsive Reporter Genes Stress Promoters Reporter ORF Stress Pathway Genotoxic stress Oxidative stress Heat shock (protein unfolding) Endoplasmic reticulum stress Inflammatory response Hypoxia Heavy metals exposure Gene. Reporters Promoters p 21, GADD 45 A, Firefly Luciferasep 53 -RE Hmox-1, Nrf 2 -RE, NQO 1 Renilla Luciferase hsp 70, HSF 1 -RE Secreted Metridia Luciferase Grp 78, XBP 1 -RE Fluorescent Proteins IL-8, NFk. B-RE Secreted Alkaline Phosphatase Grp 94, HIF 1 -RE, Hi 95 Beta-Glucoronidase MT-1, MT-2 A, MTF 1 -RE Beta-Lactamase Beta-Galactosidase Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch

Why Reporter Genes? • Why not measure stress protein levels? q Stability- rapid turnover q Throughput • Why not measure transcripts by microarray or q. PCR? q Cost q Throughput § Remember… we need to assay 1000 s of chemicals and establish dose-response information § Reporter genes meet all of the HTS requisites at the right price Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 16

What Makes a Good HTS Assay? • Miniaturization: 96 -, 384 -, 1536 -well formats q minimizes compound requirements and waste q lowers screening cost for consumables Ø dose-response and time course data • Assay Performance q Signal-to-background q Coefficient of variance q ↑ Signal/Background, ↓ CV = ↑ Z’ score • Reagent availability, cost, compatibility w/ library • Relevance Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 17
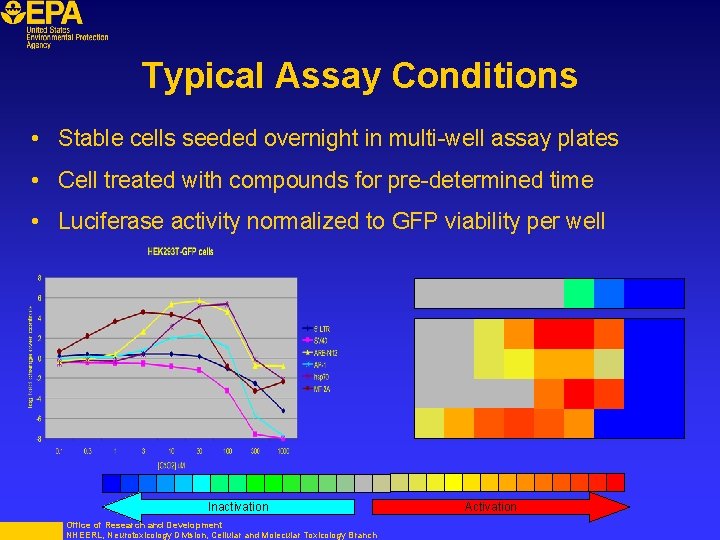
Typical Assay Conditions • Stable cells seeded overnight in multi-well assay plates • Cell treated with compounds for pre-determined time • Luciferase activity normalized to GFP viability per well Inactivation Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch Activation
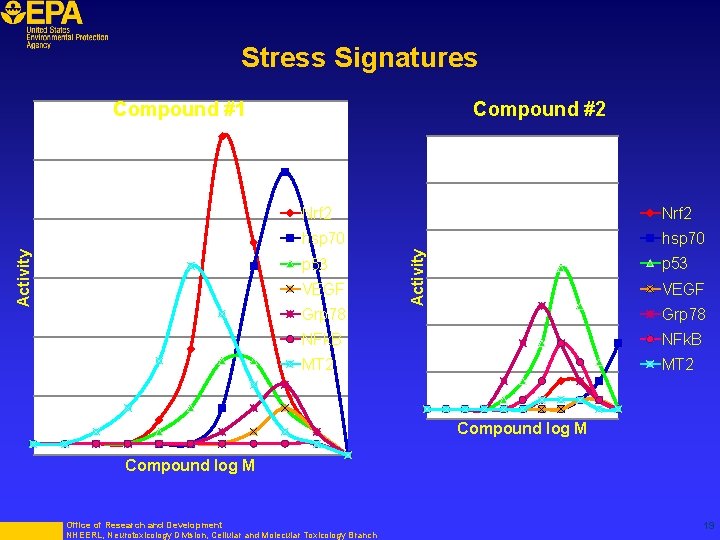
Stress Signatures Compound #2 Nrf 2 hsp 70 p 53 VEGF Grp 78 Activity Compound #1 p 53 VEGF Grp 78 NFk. B MT 2 Compound log M Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 19

Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 20

Throwing Off Pharmaco-philosophy • Disparity between the mandates of pharma industry and regulatory toxicology q Acceptance of false positive/negatives q Known targets vs. unknown mechanisms q $$$ • Much of the efforts to-date to implement HTS tox testing has adopted pharma tools and pharma thinking q Blunt tests for cytotoxicity vs. sensitive assays for “drug-able” targets q Single dose testing (usually determined by solubility) q Overly-conservative criteria for calling “hits” Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 21

Throwing Off Pharmaco-philosophy • What is needed: q Reduced reliance on loss-of-signal assays q Better understanding of mechanisms/pathways q Sensitive assays to measure mechanistic endpoints q Chemical libraries at higher concentrations q Dose-response information q Establish criteria for determining “actives” q $$$ Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 22

Chemical Library Construction • Chemicals are assembled in groups of several hundreds • Diverse chemical properties including solubility • Pharma libraries use constrained property ranges q Molecular weight q log P • DMSO is currently the solvent of choice • Compound with lowest solubility determines solubility limit for the entire library • This increases chances for false negatives Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 23
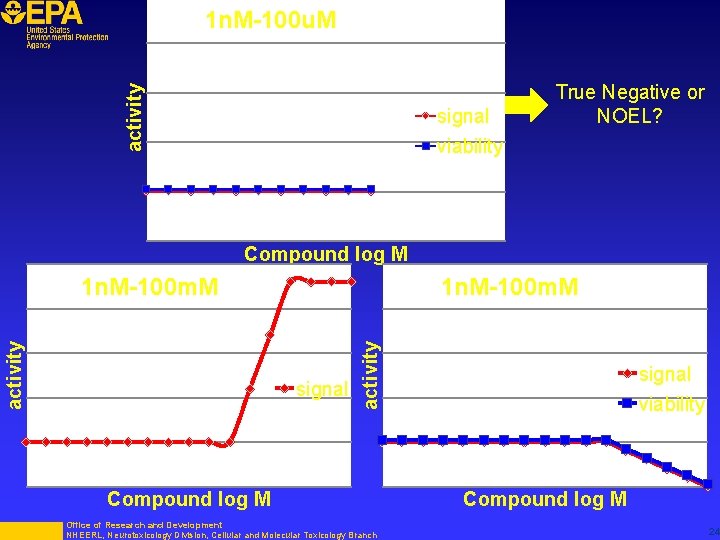
activity 1 n. M-100 u. M signal viability True Negative or NOEL? Compound log M 1 n. M-100 m. M signal activity 1 n. M-100 m. M Compound log M Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch signal viability Compound log M 24
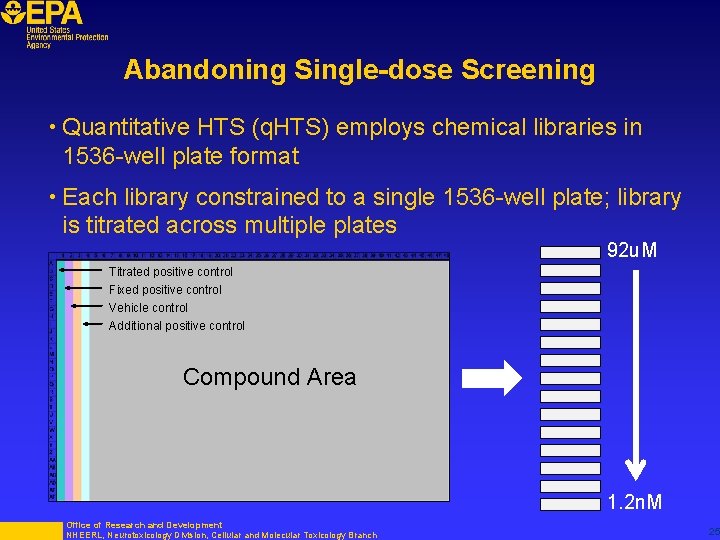
Abandoning Single-dose Screening • Quantitative HTS (q. HTS) employs chemical libraries in 1536 -well plate format • Each library constrained to a single 1536 -well plate; library is titrated across multiple plates 92 u. M Titrated positive control Fixed positive control Vehicle control Additional positive control Compound Area 1. 2 n. M Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 25
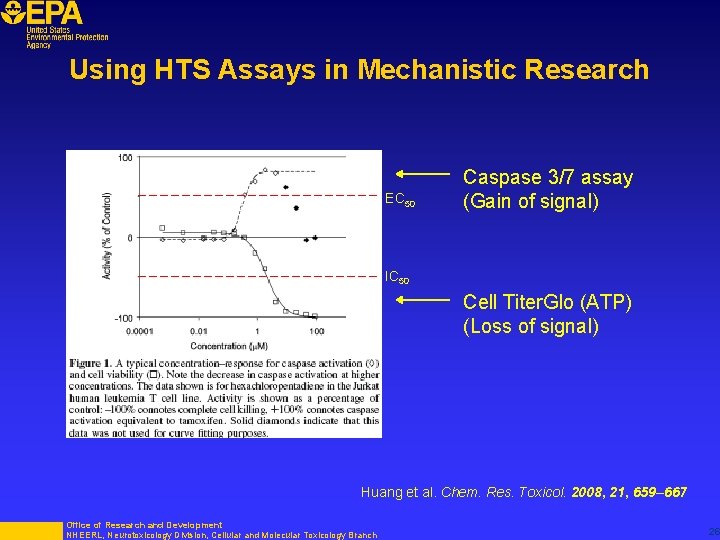
Using HTS Assays in Mechanistic Research EC 50 Caspase 3/7 assay (Gain of signal) IC 50 Cell Titer. Glo (ATP) (Loss of signal) Huang et al. Chem. Res. Toxicol. 2008, 21, 659– 667 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 26
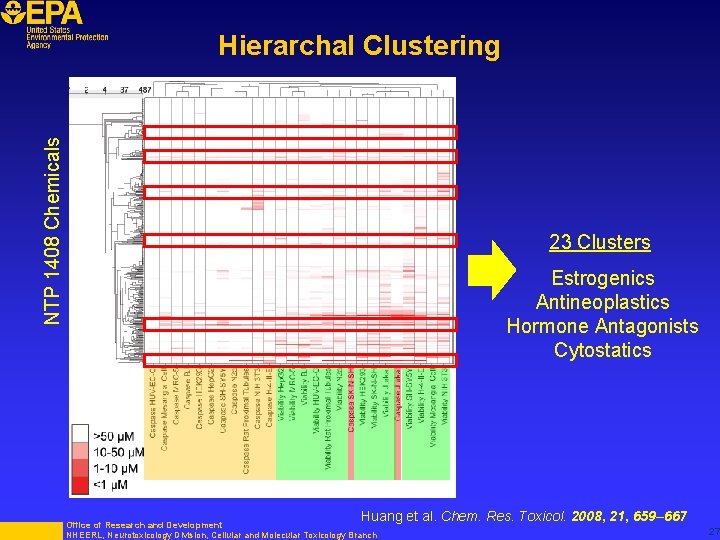
NTP 1408 Chemicals Hierarchal Clustering 23 Clusters Estrogenics Antineoplastics Hormone Antagonists Cytostatics Huang et al. Chem. Res. Toxicol. 2008, 21, 659– 667 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 27

“While the goal of the clustering is to generate a hypothesis about a compound’s specific mechanism of action, the broad nature of these cytotoxicity assays likely prevents any detailed understanding of the molecular basis of the toxic effect; the inclusion of or confirmation of activity in other, more mechanistic assays would obviously improve this aspect of the current study. ” Stress pathway assays move us a step in this direction Huang et al. Chem. Res. Toxicol. 2008, 21, 659– 667 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 28

Progress to Date • Engineered 20+ assays covering most of the key stress pathways; filling in gaps with new assays • Miniaturizing assay to 1536 -well format • Screened two assays (Nrf 2 and hsp 70) with two libraries: NTP and EPA; preliminary re-clustering • Screening additional assays in phased manner • New chemical libraries • NTP-B 1408: Summer 2009 • EPA-B 1408: Summer 2009; EPA-C 1408: Under consideration • Adding primary cells models: human hepatocytes, rodent renal proximal tubule cells, etc. 29 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch
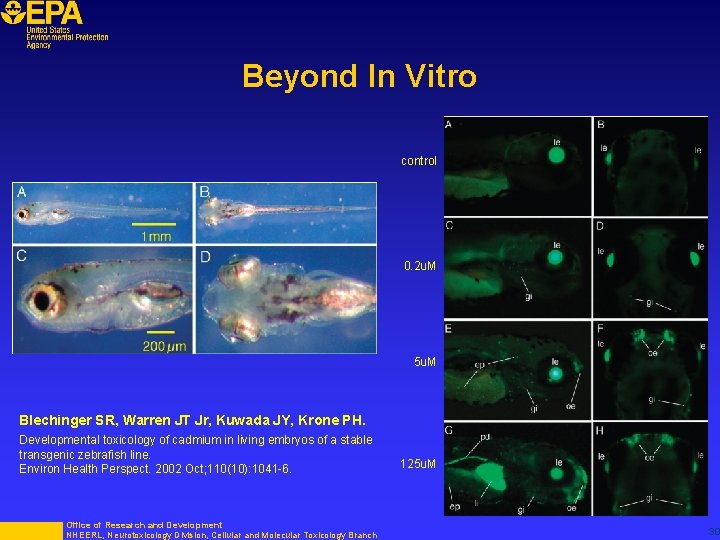
Beyond In Vitro control 0. 2 u. M 5 u. M Blechinger SR, Warren JT Jr, Kuwada JY, Krone PH. Developmental toxicology of cadmium in living embryos of a stable transgenic zebrafish line. Environ Health Perspect. 2002 Oct; 110(10): 1041 -6. Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 125 u. M 30
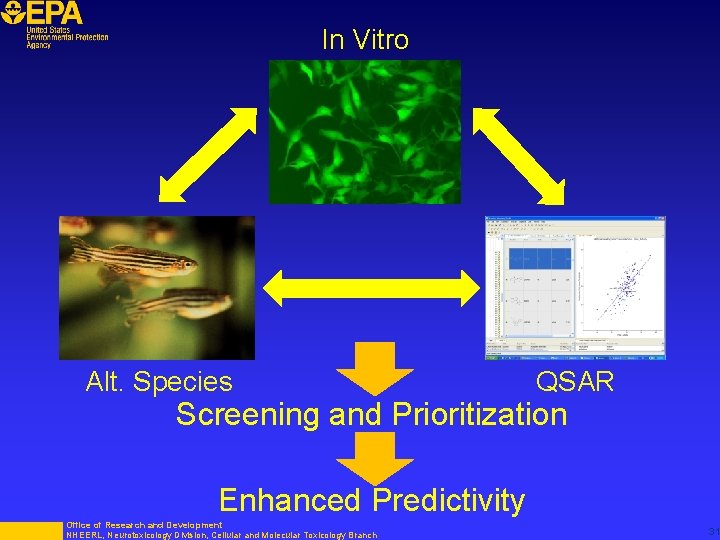
In Vitro Alt. Species QSAR Screening and Prioritization Enhanced Predictivity Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch 31

Summary and Conclusions • Stress pathway assay ensemble to generate “stress signatures” • Clustering by biological response in in vitro assays: structural similarities? ? ? • Can this type of information improve QSAR models? • Current HTS assays measure typically blunt responses: mechanisms will need further delineation • As we move forward with HTS testing, we need to move from the pharma approach to maximize information gains that will useful for toxicology 32 Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch

Acknowledgements US EPA Neurotoxicology Division Ramabhadran NIH Chemical Genomic Center Chris Austin Chun-Yang Fan Jeanene Olin Theresa Freudenrich Helen Carlsen Jim Inglese Menghang Xia Ruili Huang Sunita Shukla The Hamner Institutes Rusty Thomas US EPA, National Center for Computational Toxicology Keith Houck David Dix Office of Research and Development NHEERL, Neurotoxicology Division, Cellular and Molecular Toxicology Branch Open Biosystems (Thermo-Fisher) John Wakefield Attila Seyhan
- Slides: 34