Strengthening systems for safety monitoring for SMC in









- Slides: 14

Strengthening systems for safety monitoring for SMC in the Sahel Jean Louis Ndiaye on behalf of WARN/CARN SMC working group

Introduction • SMC implementation research phase: • over 1 million doses of SP+AQ delivered • No safety signal • Vomiting was the common Adverse drug reaction reported • No Steven Johnson Syndrome reported But if SMC is delivered at scale, • 1 Extra Pyramidal Syndrome reported will all countries be in position of monitoring Safety and detecting Serious Adverse Drug Reaction (SADR) if any ?

PV situation In West and central Africa in 2014 • PV systems were not adequately resourced for properly monitoring safety during SMC • Some countries were not member of WHO PV system (Chad, Gambia) • National Safety committees on paper only • Few countries were reporting in VIGIBASE URGENT NEED TO STRENGTEN EXISTING PV SYSTEM

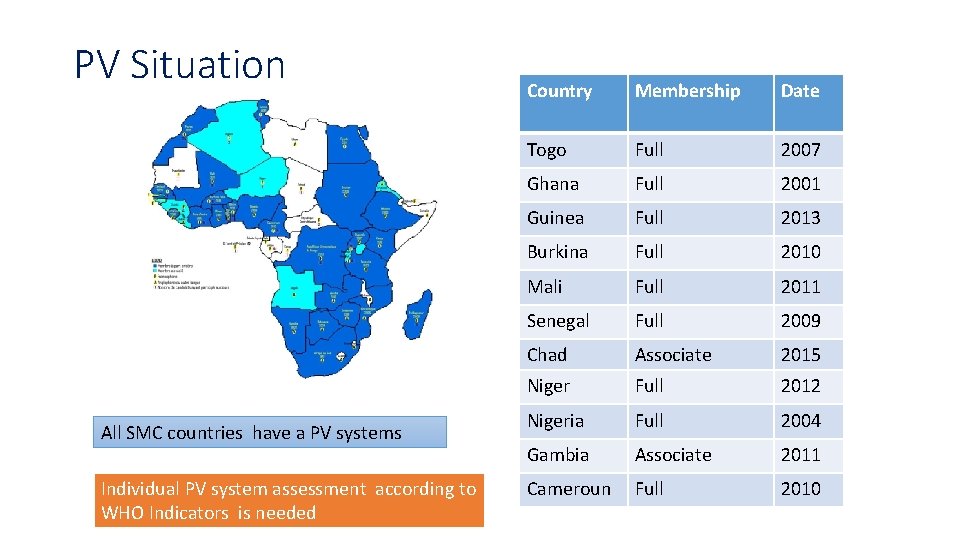
PV Situation All SMC countries have a PV systems Individual PV system assessment according to WHO Indicators is needed Country Membership Date Togo Full 2007 Ghana Full 2001 Guinea Full 2013 Burkina Full 2010 Mali Full 2011 Senegal Full 2009 Chad Associate 2015 Niger Full 2012 Nigeria Full 2004 Gambia Associate 2011 Cameroun Full 2010

Approach for strengthening the system 1. Situation analysis and needs assessment 2. Workshops for strengthening PV country expertise a) b) c) d) Geneva (Oct 2014) : Situation analysis and needs assessment Rabat (May 2015) : for strengthening PV country expertise Rabat (Feb 2016) : Lessons learned workshop Ouagadougou (Sept 2016) : Management of severe adverse reactions 3. Support for implementing more effective safety monitoring 4. Regional safety committee Collaborative approach and partnership with: WHO PV control programme and collaborative PV centres Morocco and Ghana, Malaria Consortium, CRS, UCAD, LSHTM and WHO/TDR

Support for implementing PV system for SMC 1. Action plan developed for each country to be ready for 2015 SMC - Detection, management, response, reporting and follow-up - Assessment of causality by the National Safety Committee - Reporting to UPPSALA (VIGIFLOW, VIGIBASE) 2. Job aids printed 3. In country cascade training 4. Awareness raising

Regional Safety committee - Committee composed by 4 experts in Pharmacovigilance from Morocco, Ghana, Nigeria and India - Secretariat : WHO and LSHTM representative - Role of the committee: - To analyse SMC safety data from early roll-out programmes, including the ACCESS-SMC project - To make recommendations on alternative methods of safety data collection and reporting to the WHO Advisory Committee on Safety of Medicinal Products (ACSo. MP) - First meeting in June 2016 and next meeting scheduled in March 2017 to discuss 2016 SMC safety data

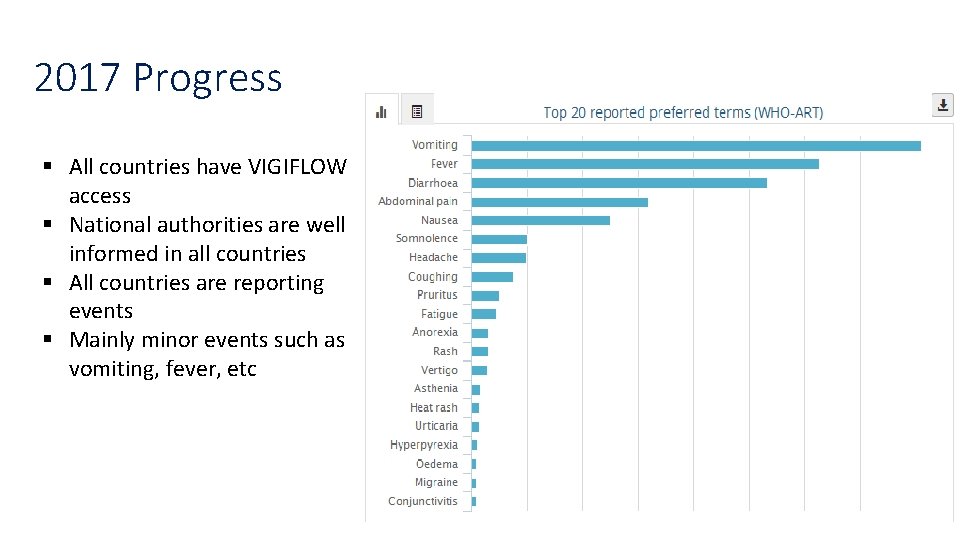
2017 Progress § All countries have VIGIFLOW access § National authorities are well informed in all countries § All countries are reporting events § Mainly minor events such as vomiting, fever, etc

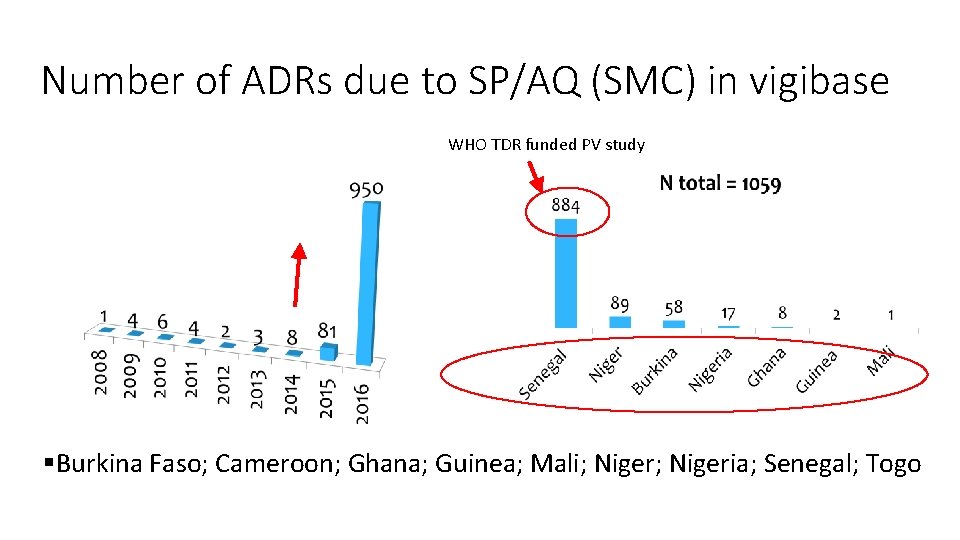
Number of ADRs due to SP/AQ (SMC) in vigibase WHO TDR funded PV study §Burkina Faso; Cameroon; Ghana; Guinea; Mali; Nigeria; Senegal; Togo

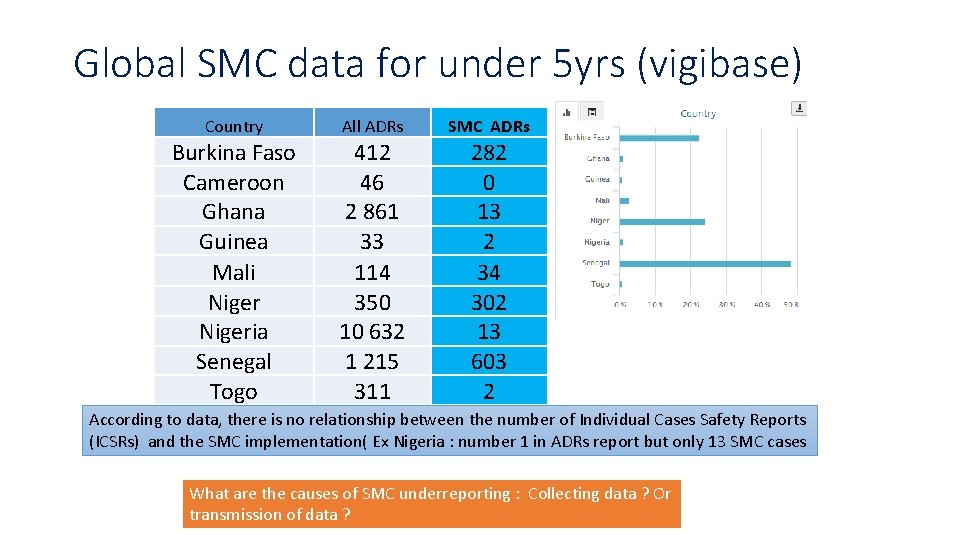
Global SMC data for under 5 yrs (vigibase) Country All ADRs SMC ADRs Burkina Faso Cameroon Ghana Guinea Mali Nigeria Senegal Togo 412 46 2 861 33 114 350 10 632 1 215 311 282 0 13 2 34 302 13 603 2 According to data, there is no relationship between the number of Individual Cases Safety Reports (ICSRs) and the SMC implementation( Ex Nigeria : number 1 in ADRs report but only 13 SMC cases What are the causes of SMC underreporting : Collecting data ? Or transmission of data ?

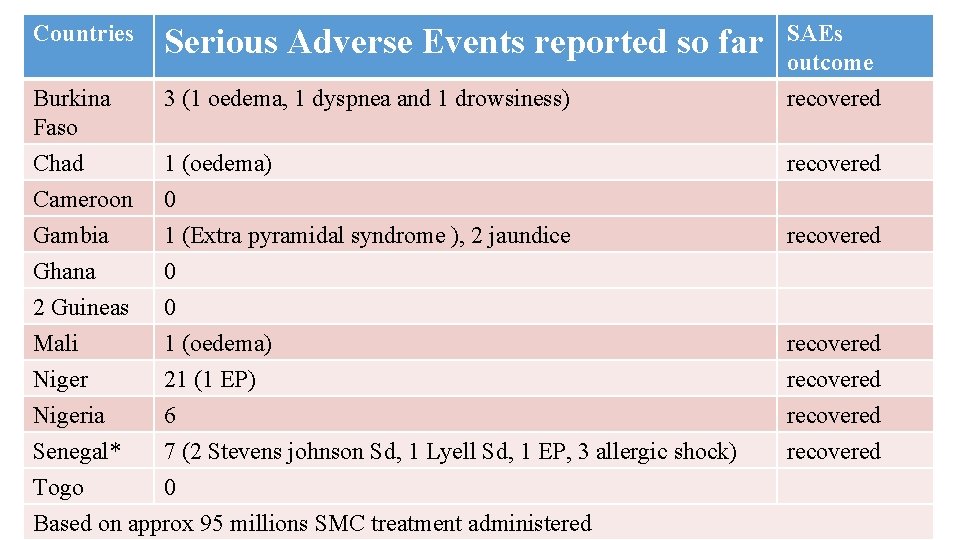
Countries Serious Adverse Events reported so far SAEs outcome Burkina Faso 3 (1 oedema, 1 dyspnea and 1 drowsiness) recovered Chad 1 (oedema) Cameroon 0 Gambia 1 (Extra pyramidal syndrome ), 2 jaundice Ghana 0 2 Guineas 0 Mali 1 (oedema) Niger 21 (1 EP) Nigeria 6 Senegal* 7 (2 Stevens johnson Sd, 1 Lyell Sd, 1 EP, 3 allergic shock) Togo 0 Based on approx 95 millions SMC treatment administered recovered recovered

Problems detected by analysing cases • Duplication of cases • Quality of the reporting form • Quality of data collected • Severity not reported • The time of onset • Causality assessment (ex: vomiting <30 min related to the taste of drugs) Need to : • Improve the countries reporting form to collect all critical data for analysis • Develop methods at country level to detect duplication • Train PV inputability committee on causality assessment

Conclusion • SMC safe and seems well tolerated • SMC is an opportunity to strengthen PV system in African countries • Since 2014 progress were done in SMC countries but more need to be done • Additional resources are needed for Countries to adequately monitoring safety during Mass Drug Administration campaign such as SMC • Public health programmes should include funds for PV and PV centres in their budget • New technologies such as smartphones can be used to establish safety profile for SMC or other new public health interventions

Thanks • • • Communities WHO TDR National PV centres NMCPs, Regional PV centres , Morocco, Ghana WHO Safety department PMI USAID, UNITAID, Unicef, GF, WB Wellcome trust UCAD, LSHTM, MRTC, CERMES, IRSS, UGAN, MRC