STRENGTH OF ACIDS AND BASES SALTS AND BUFFERS

STRENGTH OF ACIDS AND BASES & SALTS AND BUFFERS Chapter 19. 3 and 19. 5 Notes #21
Part 1: Strengths of Acids and Bases

How is there is a difference? • Citrus fruits contain citric acid that we can eat. • Industrial companies use sulfuric acid that can cause severe burns to the skin. • Why are some acids weak and some acids strong?
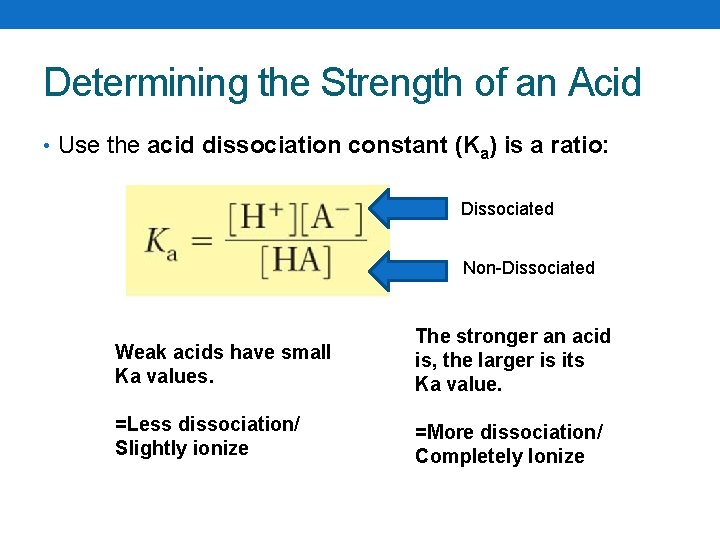
Determining the Strength of an Acid • Use the acid dissociation constant (Ka) is a ratio: Dissociated Non-Dissociated Weak acids have small Ka values. The stronger an acid is, the larger is its Ka value. =Less dissociation/ Slightly ionize =More dissociation/ Completely Ionize

Larger Ka = Stronger Acid
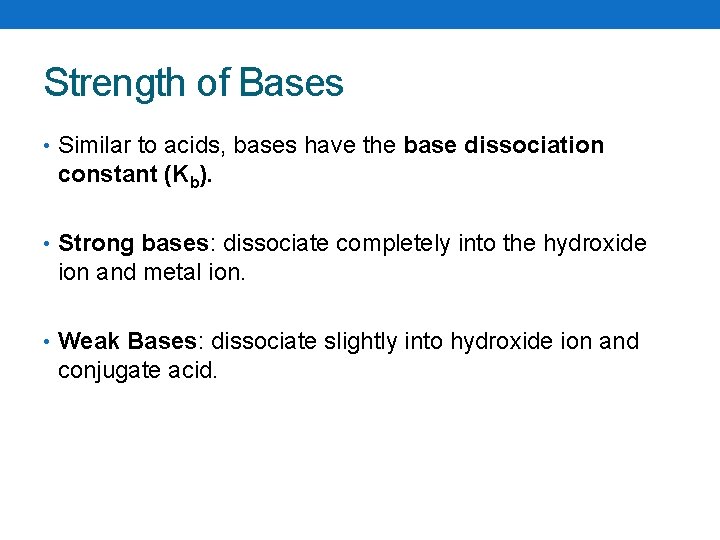
Strength of Bases • Similar to acids, bases have the base dissociation constant (Kb). • Strong bases: dissociate completely into the hydroxide ion and metal ion. • Weak Bases: dissociate slightly into hydroxide ion and conjugate acid.

Table of Relative Strengths

a. Explain In the graph for the strong acid, why are the heights of H 3 O+ and A- bars the same as the height of the HA bar? a. Inferring In the graph of the weak acid, why is the height of the H 3 O+ the same as the distance from the top of the second HA bar to the dotted line?
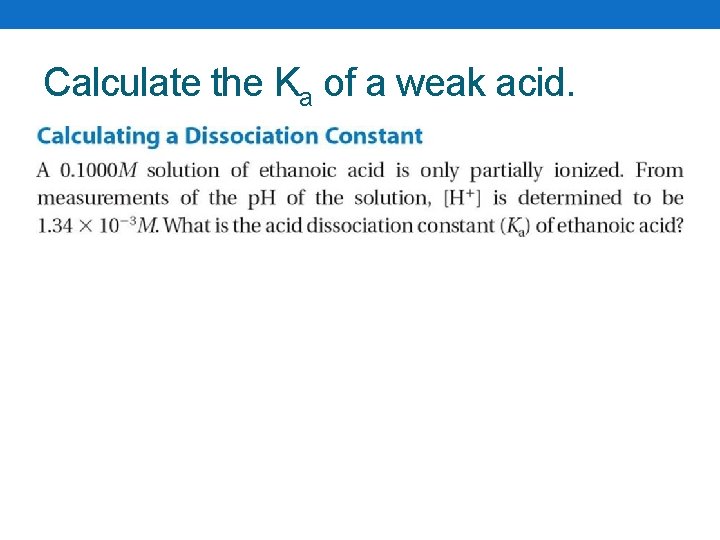
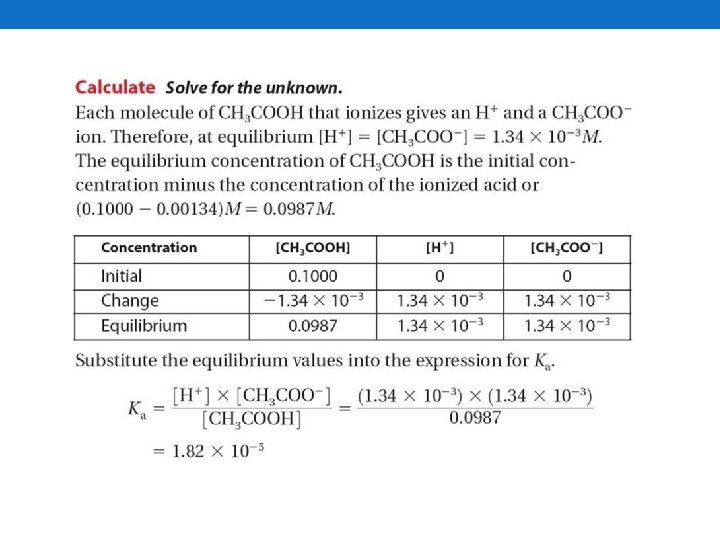
Calculate the Ka of a weak acid.


Part 2: Buffers and Salts

What is a salt? • It is a compound that contains an anion from an acid and a cation from a base. • They form as a result of an acid-base neutralization reaction. • Not just table salt! Although, this is also considered a salt. HCl + Na. OH Na. Cl + H 2 O KOH + HNO 3 KNO 3 + H 2 O

Solutions made from these salts can be neutral, acidic, or basic.

Universal indicator solution has been added to each of these 0. 10 M aqueous salt solutions. NH 4 Cl p. H 5. 3 Na. Cl p. H 7 CH 3 COONa p. H 5. 3 Universal Indicator: Like a liquid p. H strip. Changes the solution to a color to tell you the p. H.

Buffers • A Buffer is made by making a solution that contains a mixture of: • a weak acid and its salt or • a weak base and its salt. Characteristics of a Buffer Solution: • The p. H of a buffer remains relatively constant when small amounts of acid or base are added. • The buffer capacity is the amount of acid or base that can be added to a buffer solution before a significant change in p. H occurs.

Buffers Contd… • Buffers have neutralizing “powers”. • In a sense, it eats up the acid or base you have to remain at a constant p. H. • Eventually, the buffer will be used up, and the p. H can then change dramatically.

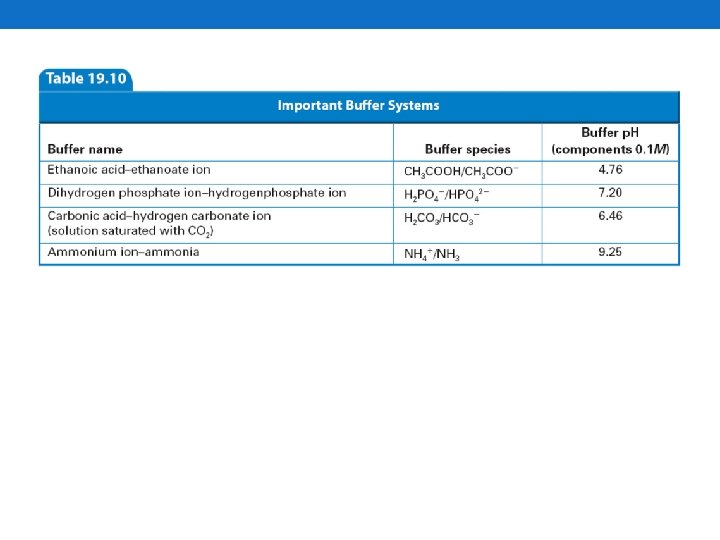
How to choose a Buffer • The p. H of the acid or base being added to the buffer should be within the p. H range of the buffer. Buffer System p. H Range Monohydrogen phosphate/dihydrogen phosphate 6. 1 -7. 4 Ethanoate/ ethanoic acid 3. 7 -5. 6 Carbonate/ hydrogen carbonate 9. 2 -11. 0 Phosphate/ monohydrogen phosphate 11. 0 -12. 0 Question: Which of these buffers would be effective at p. H 5. 0?
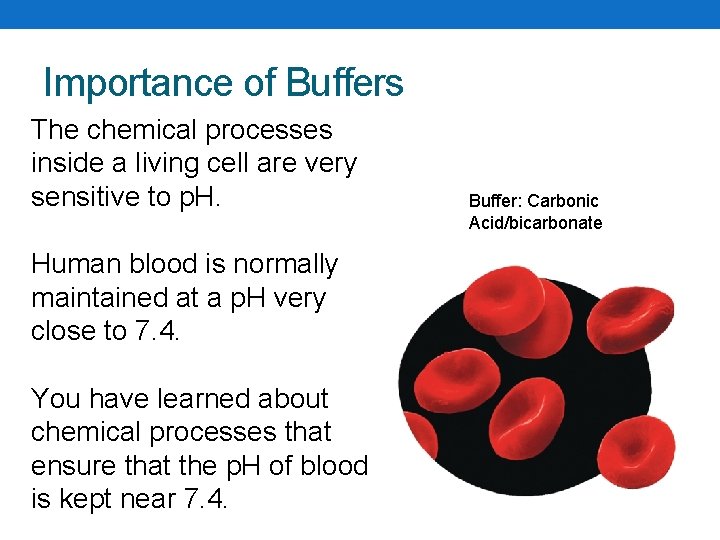
Importance of Buffers The chemical processes inside a living cell are very sensitive to p. H. Human blood is normally maintained at a p. H very close to 7. 4. You have learned about chemical processes that ensure that the p. H of blood is kept near 7. 4. Buffer: Carbonic Acid/bicarbonate

The Buffer in Our Blood • An abnormal p. H level of the blood can be due to several factors: overexerting your body through exercise, improper diet, drug consumption, and other biological factors such as kidney failure (since the kidneys are responsible for removing excess H+ ions and other components of the p. H buffer). • Below 6. 8 (acidosis) or above 7. 8 (alkalosis) will result in death.
- Slides: 21