Strength of Acids and Bases Do they ionize

Strength of Acids and Bases Do they ionize 100%?

Strong Acids : Give up H+ easily Dissociate completely (100%) in water HCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 3

Weak acids: (all others) Hold onto H+ Few molecules dissociate Ex: HC 2 H 3 O 2 Strong/Weak Acid Animation http: //educypedia. karadimov. info/library/acid 13. swf

Let’s examine the behavior of an acid, HA, in aqueous solution. HA What happens to the HA molecules in solution?
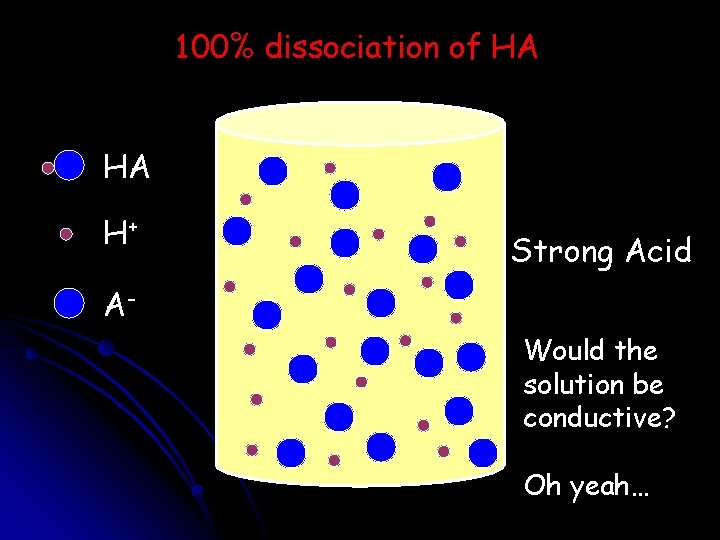
100% dissociation of HA HA H+ Strong Acid AWould the solution be conductive? Oh yeah…
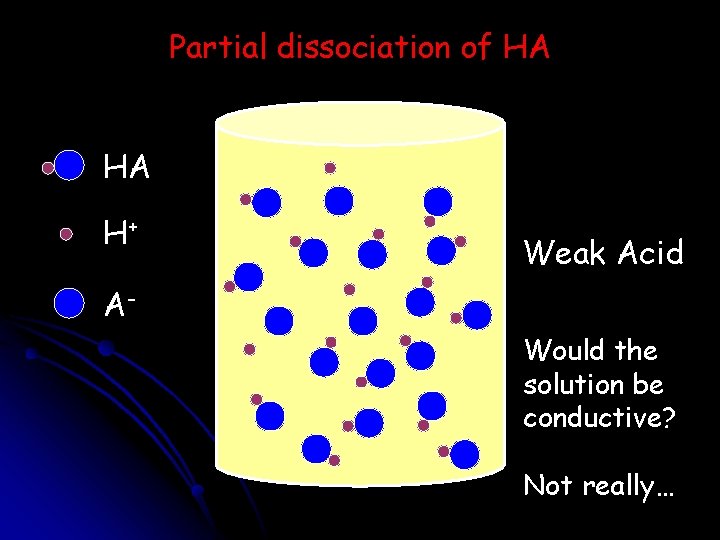
Partial dissociation of HA HA H+ Weak Acid AWould the solution be conductive? Not really…
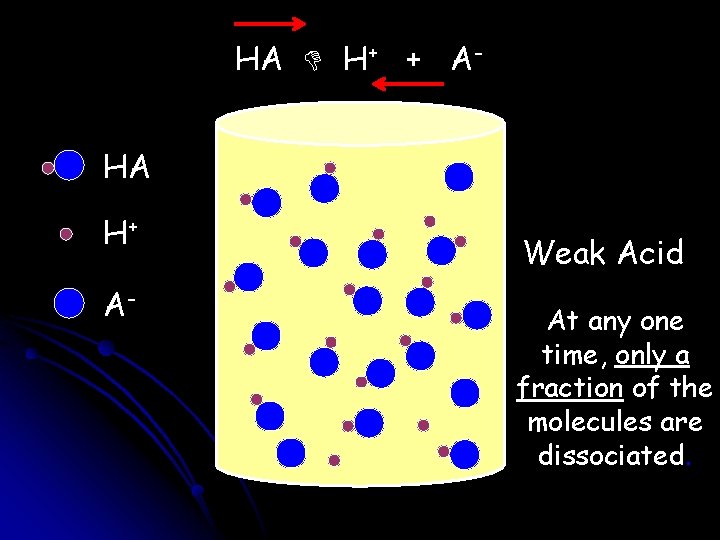
HA H+ + AHA H+ A- Weak Acid At any one time, only a fraction of the molecules are dissociated.

Strong Bases: Dissociate completely (100%) in water - Group I metal hydroxides (Na. OH, Li. OH, etc. ) - Some Group II metal hydroxides Ca(OH)2, Ba(OH)2, Sr(OH)2 Weak Bases Only a few ions dissociate Ex: NH 3 (ammonia)

Strength and Reactivity l Acids/bases of the same initial molar concentration can react differently and conduct electricity differently if one is weak and the other strong. l Ex: 2 M HCl Strong Acid, very conductive very reactive 2 M HC 2 H 3 O 2 Weak Acid Weak Conduction Salad Dressing!!!

Conjugate Acid/Base Pairs Strong acid will have a weak conjugate base l Strong base will have a weak conjugate acid l
Hydrolysis l Opposite reaction to neutralization Salt + Water Acid + Base

Parent Acid/Base l If you know the salt involved you should be able to determine which acid and base it would form if water is added. Salt + Water Acid + Base Ex: Na. Cl with water (HOH) would form HCl and Na. OH

You Try It l Name the “parent” acid and base that would be produced from these salts. l Ex: Potassium chloride Magnesium carbonate
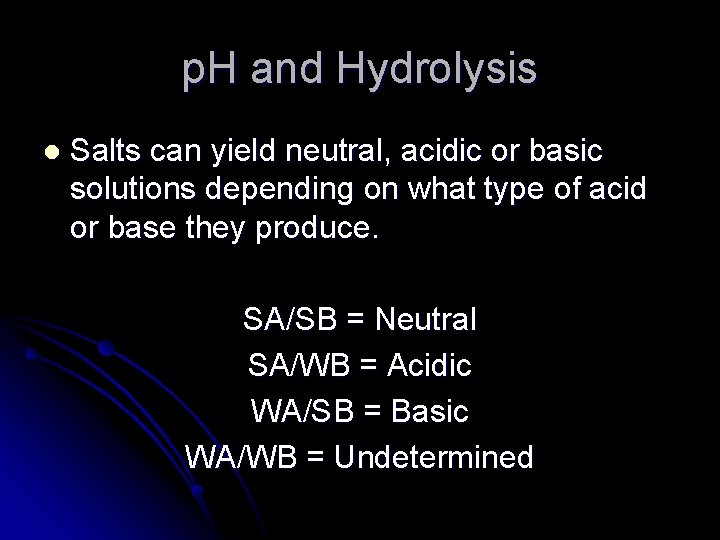
p. H and Hydrolysis l Salts can yield neutral, acidic or basic solutions depending on what type of acid or base they produce. SA/SB = Neutral SA/WB = Acidic WA/SB = Basic WA/WB = Undetermined

The Acid and Base Dissociation Constant, Ka & Kb
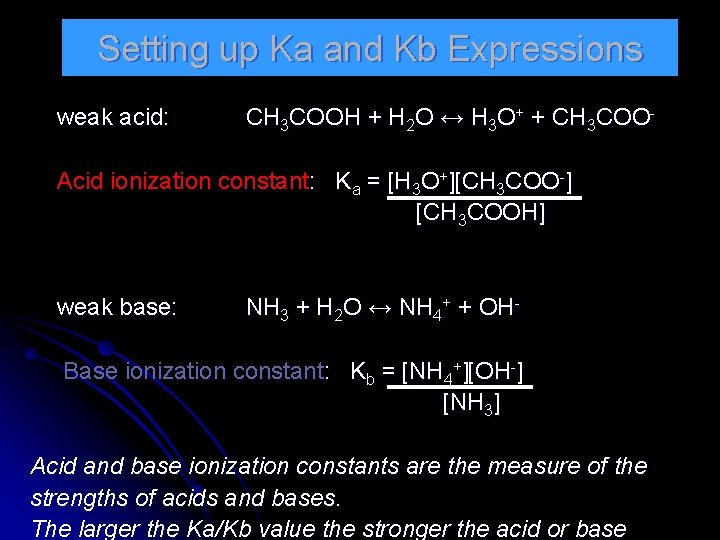
Setting up Ka and Kb Expressions weak acid: CH 3 COOH + H 2 O ↔ H 3 O+ + CH 3 COO- Acid ionization constant: Ka = [H 3 O+][CH 3 COO-] [CH 3 COOH] weak base: NH 3 + H 2 O ↔ NH 4+ + OH- Base ionization constant: Kb = [NH 4+][OH-] [NH 3] Acid and base ionization constants are the measure of the strengths of acids and bases. The larger the Ka/Kb value the stronger the acid or base


Ka l Weak acids: l only ionize to a small extent l come to a state of chemical equilibrium. l Determine how much it ionizes by calculating the equilibrium constant (Ka) l Larger Ka: l stronger acid l more ions found in solution l more easily donate a proton

HCOOH (aq) + H 2 O (l) < -- > H 3 O+ (aq) + HCOO- (aq) l Ka = [H 3 O+][HCOO-] [HCOOH] l Notice how the Ka ignores the water since we are dealing with dilute solutions of acids, water is considered a constant and doesn’t have a concentration value

Practice: 1. A solution of a weak acid, “HA”, is made up to be 0. 15 M. Its p. H was found to be 2. 96. Calculate the value of Ka. l Steps to follow: 1. 2. 3. 4. Write balanced equation Calculate [H+] using 10 -p. H Set up chart for equilibrium (ICE) Solve using Ka expression
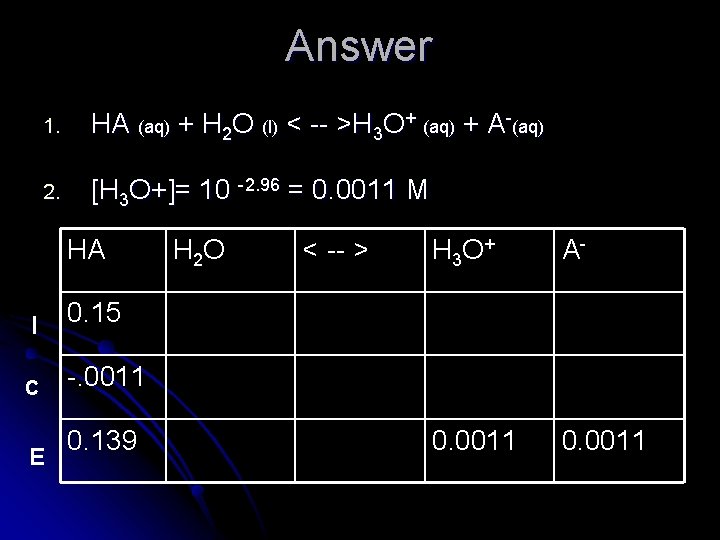
Answer 1. HA (aq) + H 2 O (l) < -- >H 3 O+ (aq) + A-(aq) 2. [H 3 O+]= 10 -2. 96 = 0. 0011 M HA I C E H 2 O < -- > H 3 O + A- 0. 0011 0. 15 -. 0011 0. 139
![4. Ka = [H 3 O+][ A-] [HA] = [0. 0011] [0. 139] = 4. Ka = [H 3 O+][ A-] [HA] = [0. 0011] [0. 139] =](http://slidetodoc.com/presentation_image_h/d4023d0a4d9b6b055541d24a4aa575e3/image-22.jpg)
4. Ka = [H 3 O+][ A-] [HA] = [0. 0011] [0. 139] = 8. 7 x 10 -6

Percent Ionization The fraction of acid molecules that dissociate compared with the initial concentration of the acid. Percent Ionization = [H 3 O+] x 100% [HA i] For the previous question: Percent Ionization = [0. 0011] x 100% =0. 73 % [0. 15]

Practice: l Ka, for a hypothetical weak acid, HA, at 25°C is 2. 2 x 10 -4. a) Calculate [H 3 O+] of a 0. 20 M solution of HA. b) Calculate the percent ionization of HA.
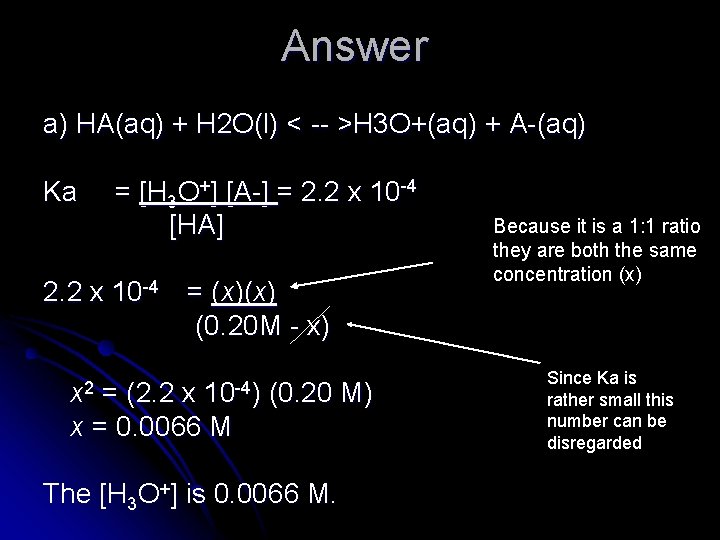
Answer a) HA(aq) + H 2 O(l) < -- >H 3 O+(aq) + A-(aq) Ka = [H 3 O+] [A-] = 2. 2 x 10 -4 [HA] 2. 2 x 10 -4 = (x)(x) (0. 20 M - x) x 2 10 -4) = (2. 2 x x = 0. 0066 M (0. 20 M) The [H 3 O+] is 0. 0066 M. Because it is a 1: 1 ratio they are both the same concentration (x) Since Ka is rather small this number can be disregarded

b) % ionization = 0. 0066 M x 100% = 3. 3% 0. 20 M
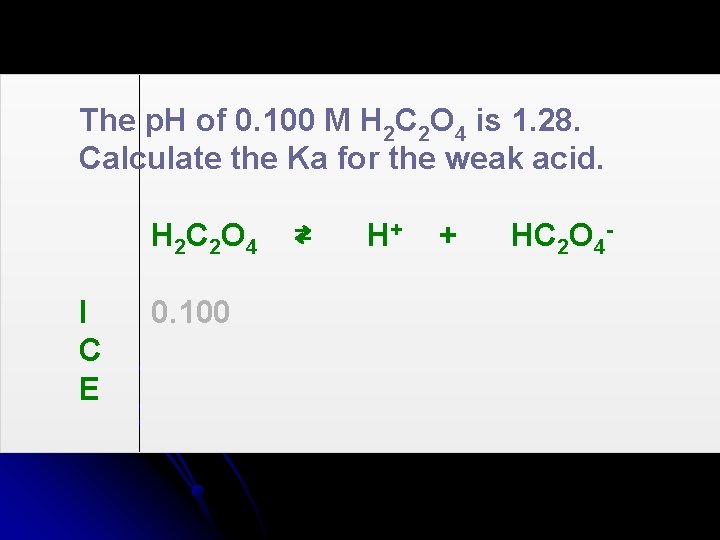
The p. H of 0. 100 M H 2 C 2 O 4 is 1. 28. Calculate the Ka for the weak acid. H 2 C 2 O 4 I C E 0. 100 ⇄ H+ + HC 2 O 4 -
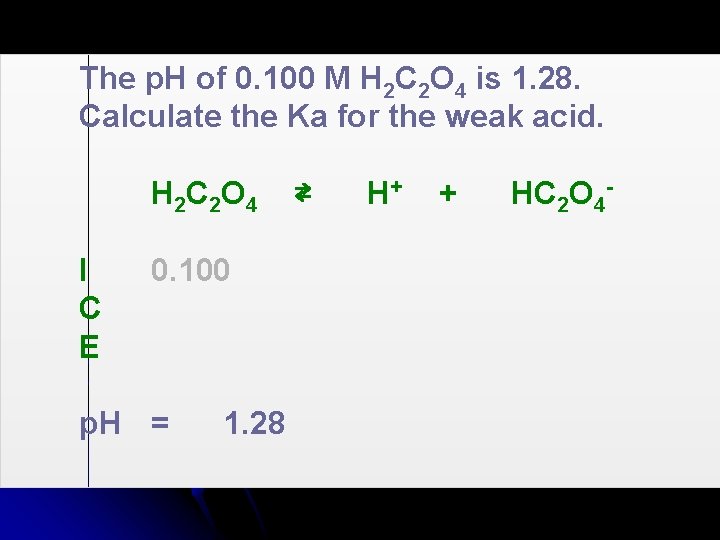
The p. H of 0. 100 M H 2 C 2 O 4 is 1. 28. Calculate the Ka for the weak acid. H 2 C 2 O 4 I C E 0. 100 p. H = 1. 28 ⇄ H+ + HC 2 O 4 -
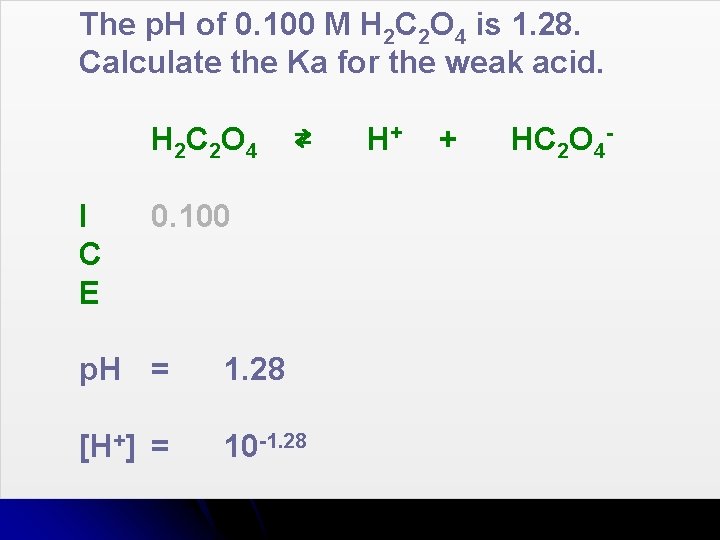
The p. H of 0. 100 M H 2 C 2 O 4 is 1. 28. Calculate the Ka for the weak acid. H 2 C 2 O 4 I C E ⇄ 0. 100 p. H = 1. 28 [H+] = 10 -1. 28 H+ + HC 2 O 4 -
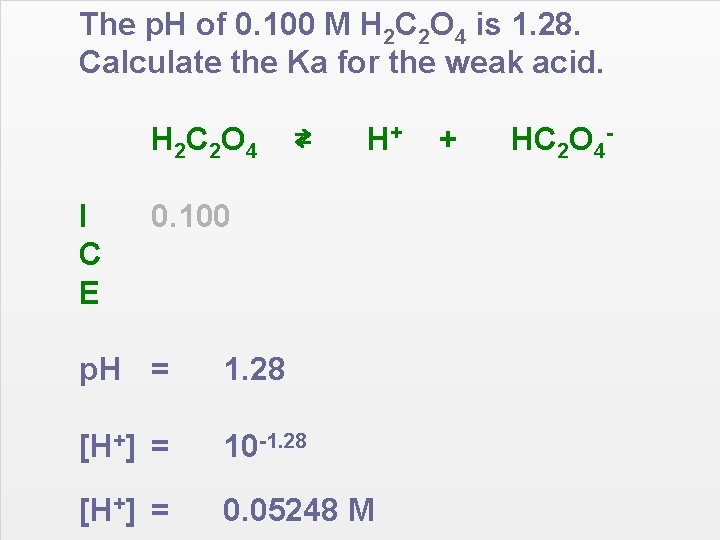
The p. H of 0. 100 M H 2 C 2 O 4 is 1. 28. Calculate the Ka for the weak acid. H 2 C 2 O 4 I C E ⇄ H+ 0. 100 p. H = 1. 28 [H+] = 10 -1. 28 [H+] = 0. 05248 M + HC 2 O 4 -
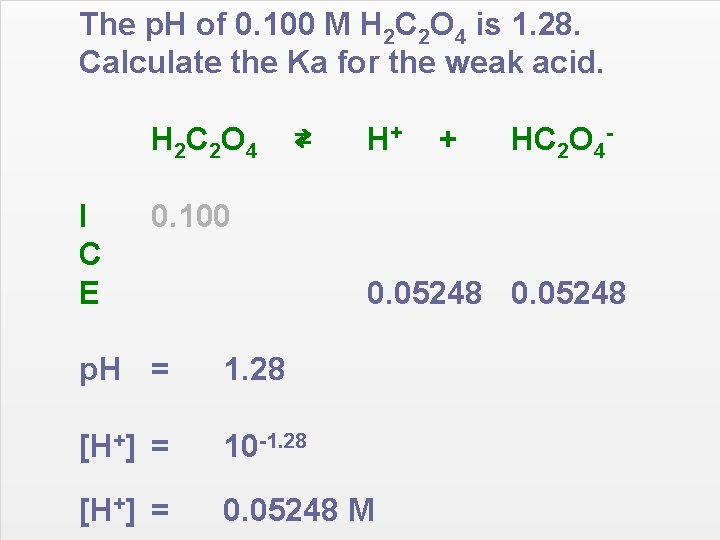
The p. H of 0. 100 M H 2 C 2 O 4 is 1. 28. Calculate the Ka for the weak acid. H 2 C 2 O 4 I C E ⇄ H+ + HC 2 O 4 - 0. 100 0. 05248 p. H = 1. 28 [H+] = 10 -1. 28 [H+] = 0. 05248 M
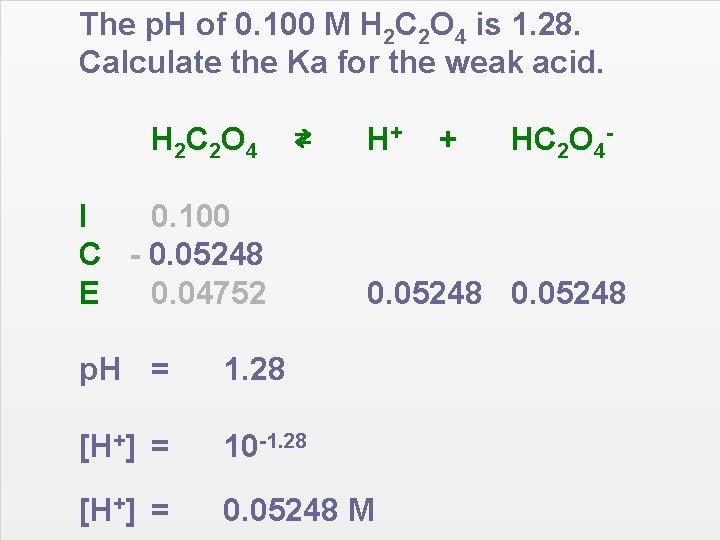
The p. H of 0. 100 M H 2 C 2 O 4 is 1. 28. Calculate the Ka for the weak acid. H 2 C 2 O 4 ⇄ I 0. 100 C - 0. 05248 E 0. 04752 H+ + HC 2 O 4 - 0. 05248 p. H = 1. 28 [H+] = 10 -1. 28 [H+] = 0. 05248 M
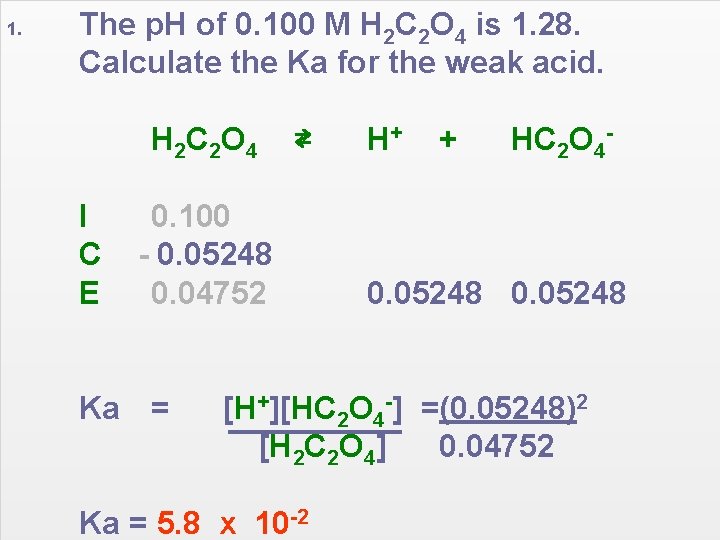
1. The p. H of 0. 100 M H 2 C 2 O 4 is 1. 28. Calculate the Ka for the weak acid. H 2 C 2 O 4 I C E ⇄ 0. 100 - 0. 05248 0. 04752 Ka = H+ + HC 2 O 4 - 0. 05248 [H+][HC 2 O 4 -] =(0. 05248)2 [H 2 C 2 O 4] 0. 04752 Ka = 5. 8 x 10 -2

Kb l When using weak bases, the same rules apply as with weak acids, except you are solving for p. OH and using [OH-]
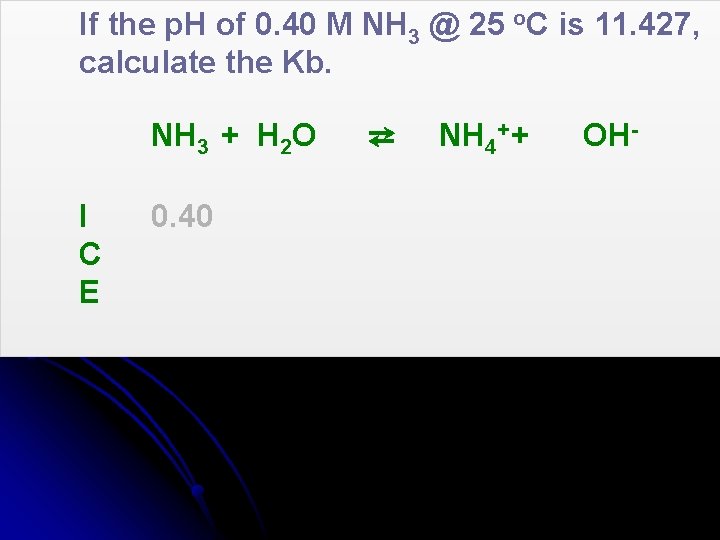
If the p. H of 0. 40 M NH 3 @ 25 o. C is 11. 427, calculate the Kb. NH 3 + H 2 O I C E 0. 40 ⇄ NH 4+ + OH-
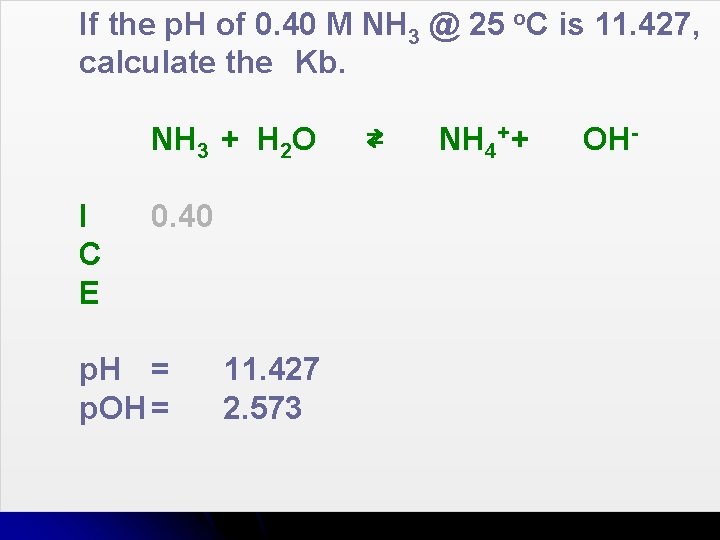
If the p. H of 0. 40 M NH 3 @ 25 o. C is 11. 427, calculate the Kb. NH 3 + H 2 O I C E 0. 40 p. H = p. OH = 11. 427 2. 573 ⇄ NH 4+ + OH-
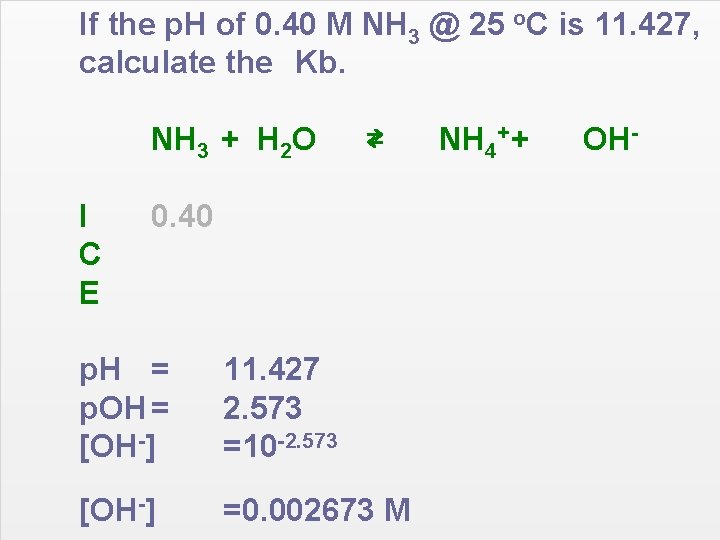
If the p. H of 0. 40 M NH 3 @ 25 o. C is 11. 427, calculate the Kb. NH 3 + H 2 O I C E ⇄ 0. 40 p. H = p. OH = [OH-] 11. 427 2. 573 =10 -2. 573 [OH-] =0. 002673 M NH 4+ + OH-
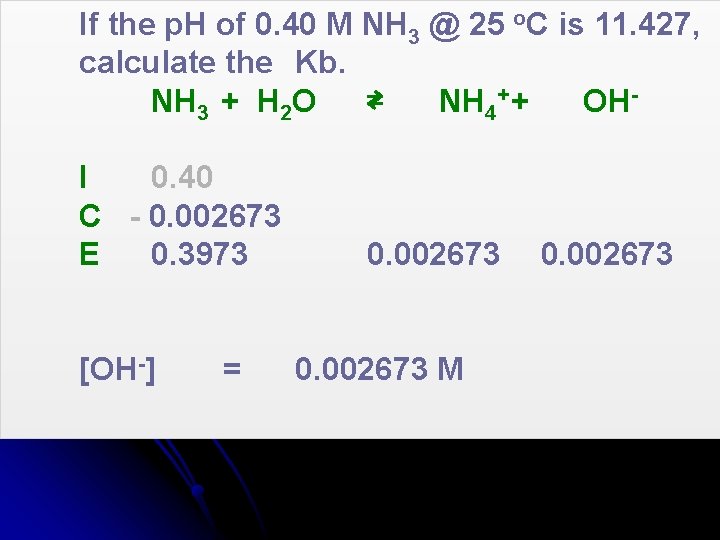
If the p. H of 0. 40 M NH 3 @ 25 o. C is 11. 427, calculate the Kb. NH 3 + H 2 O ⇄ NH 4+ + OHI 0. 40 C - 0. 002673 E 0. 3973 [OH-] = 0. 002673 M 0. 002673
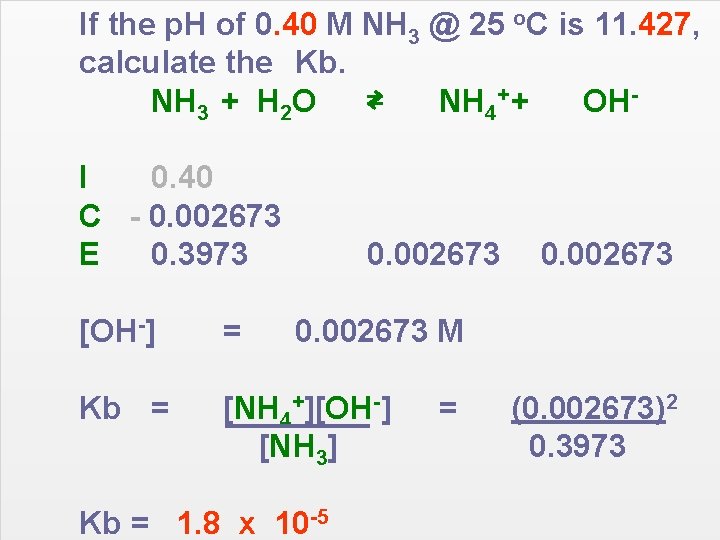
If the p. H of 0. 40 M NH 3 @ 25 o. C is 11. 427, calculate the Kb. NH 3 + H 2 O ⇄ NH 4+ + OHI 0. 40 C - 0. 002673 E 0. 3973 0. 002673 [OH-] = Kb = [NH 4+][OH-] [NH 3] 0. 002673 M Kb = 1. 8 x 10 -5 = (0. 002673)2 0. 3973

The p. H of 0. 20 M Na. CN is 11. 456, calculate the Kb for CN-.
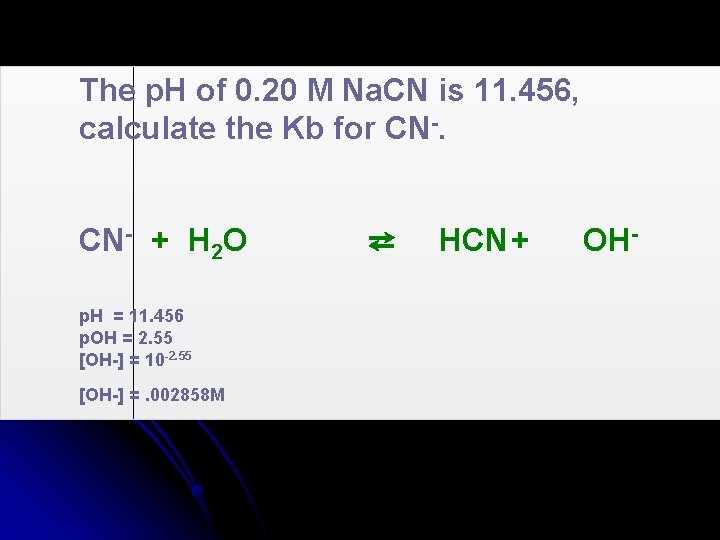
The p. H of 0. 20 M Na. CN is 11. 456, calculate the Kb for CN- + H 2 O p. H = 11. 456 p. OH = 2. 55 [OH-] = 10 -2. 55 [OH-] =. 002858 M ⇄ HCN+ OH-
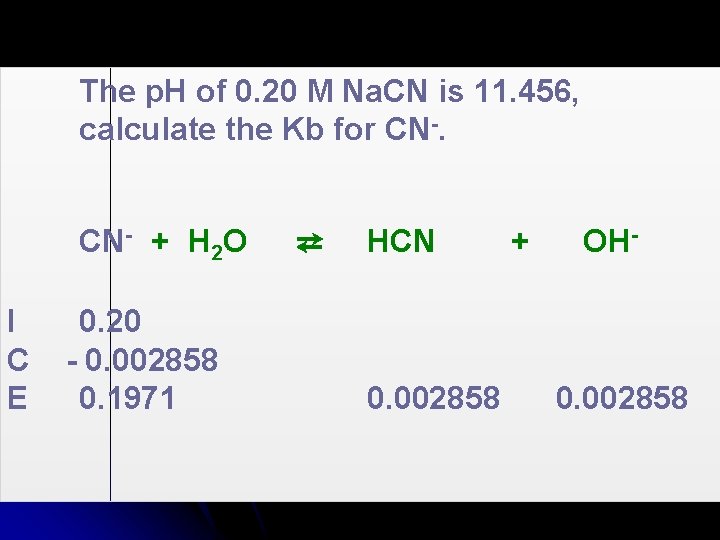
The p. H of 0. 20 M Na. CN is 11. 456, calculate the Kb for CN- + H 2 O I C E 0. 20 - 0. 002858 0. 1971 ⇄ HCN 0. 002858 + OH- 0. 002858
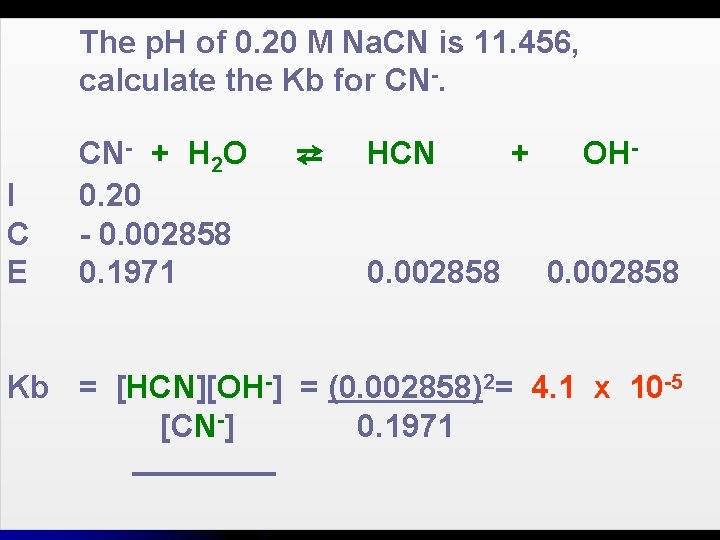
The p. H of 0. 20 M Na. CN is 11. 456, calculate the Kb for CN-. I C E CN- + H 2 O 0. 20 - 0. 002858 0. 1971 ⇄ HCN 0. 002858 + OH- 0. 002858 Kb = [HCN][OH-] = (0. 002858)2= 4. 1 x 10 -5 [CN-] 0. 1971
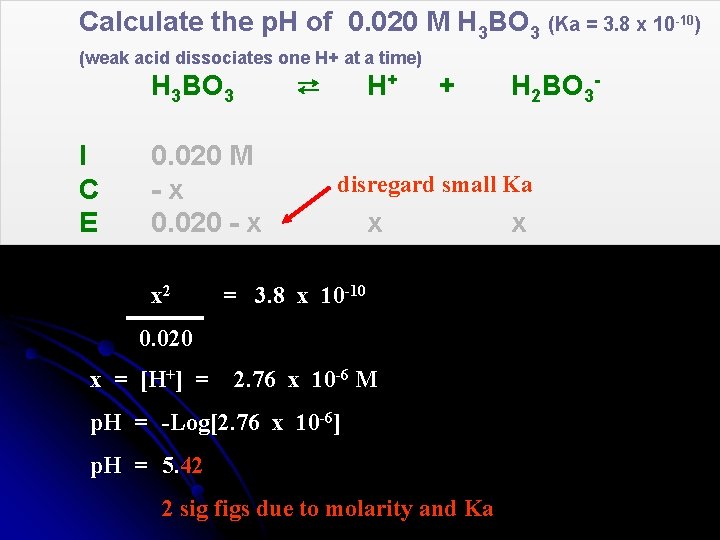
Calculate the p. H of 0. 020 M H 3 BO 3 (Ka = 3. 8 x 10 -10) (weak acid dissociates one H+ at a time) H 3 BO 3 ⇄ H+ I C E 0. 020 M -x 0. 020 - x x 2 + disregard small Ka x = 3. 8 x 10 -10 0. 020 x = [H+] = H 2 BO 3 - 2. 76 x 10 -6 M p. H = -Log[2. 76 x 10 -6] p. H = 5. 42 2 sig figs due to molarity and Ka x
- Slides: 44