Strategic Choice of ARBS To Provide Optimal Blood

Strategic Choice of ARB’S To Provide Optimal Blood Pressure Management and Reduction of Cardiovascular Events Focus On Candesartan Masrul Syafri Bagian Kardiologi & Kedokteran Vaskular FK Unand-RSUP DR. M. Djamil Padang

‘Controlling blood pressure with medication is unquestionably one of the most cost-effective methods of reducing premature CV morbidity and mortality’ Elliott. J Clin Hypertens 2003; 5(Suppl. 2): 3– 13

WHAT GUIDELINE SAY’S - JNC 8 GUIDELINES
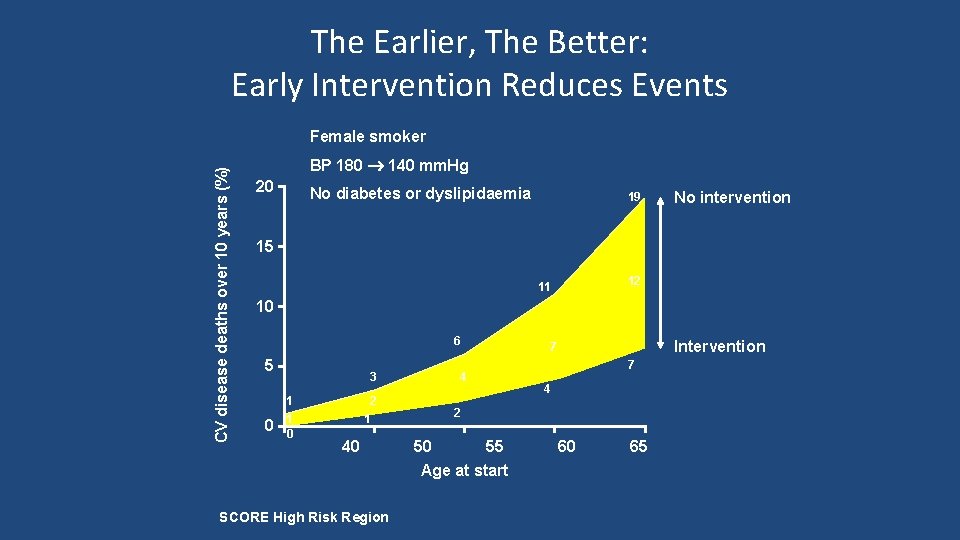
The Earlier, The Better: Early Intervention Reduces Events CV disease deaths over 10 years (%) Female smoker BP 180 140 mm. Hg 20 No diabetes or dyslipidaemia 19 No intervention 15 12 11 10 6 5 0 3 1 1 0 2 1 40 SCORE High Risk Region 4 Intervention 7 7 4 2 50 55 Age at start 60 65

OPTIMAL BLOOD PRESSURE MANAGEMENT UPDATED GUIDELINES Publication on JAMA ( The Journal of the American Medical Association ) JAMA February 5, 2014 Volume 311, Number 5

THE DIFFERENCES
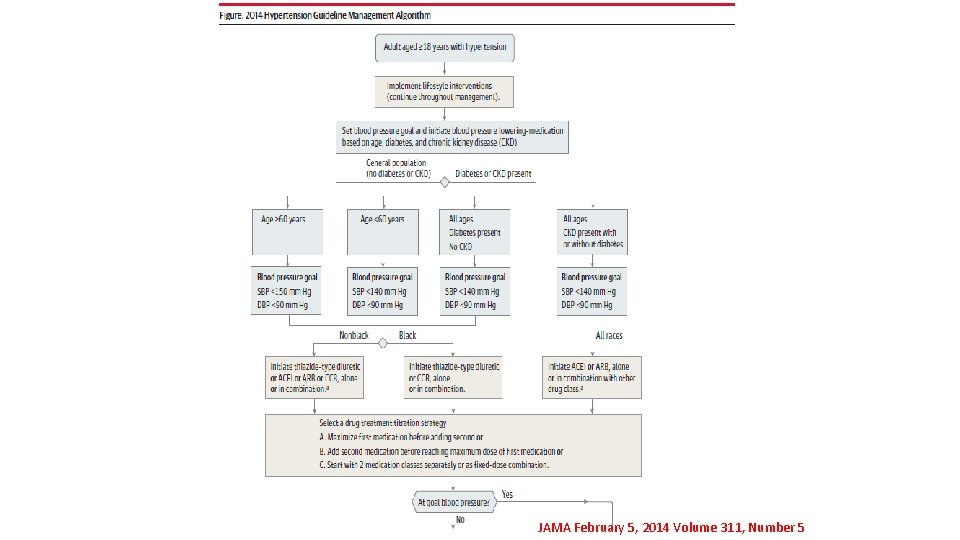
JAMA February 5, 2014 Volume 311, Number 5
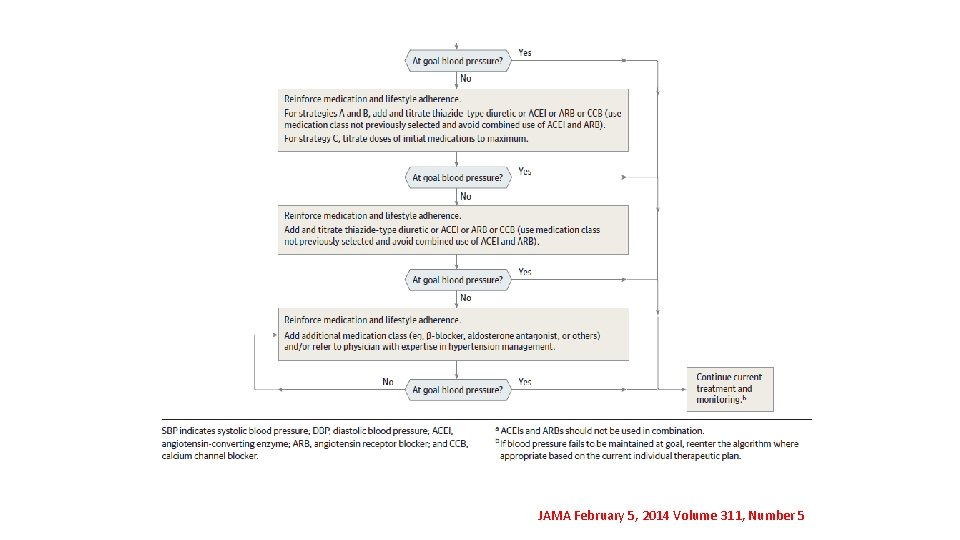
JAMA February 5, 2014 Volume 311, Number 5
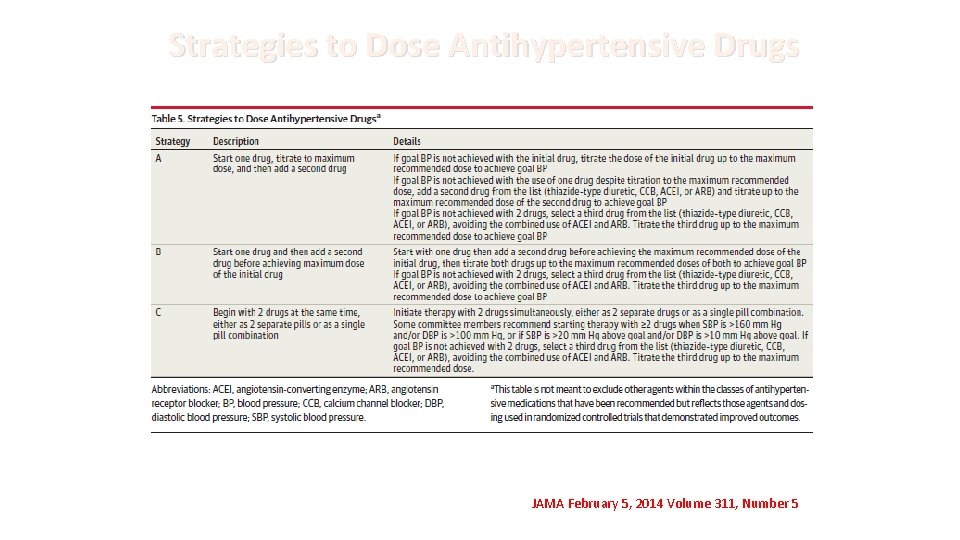
Strategies to Dose Antihypertensive Drugs JAMA February 5, 2014 Volume 311, Number 5
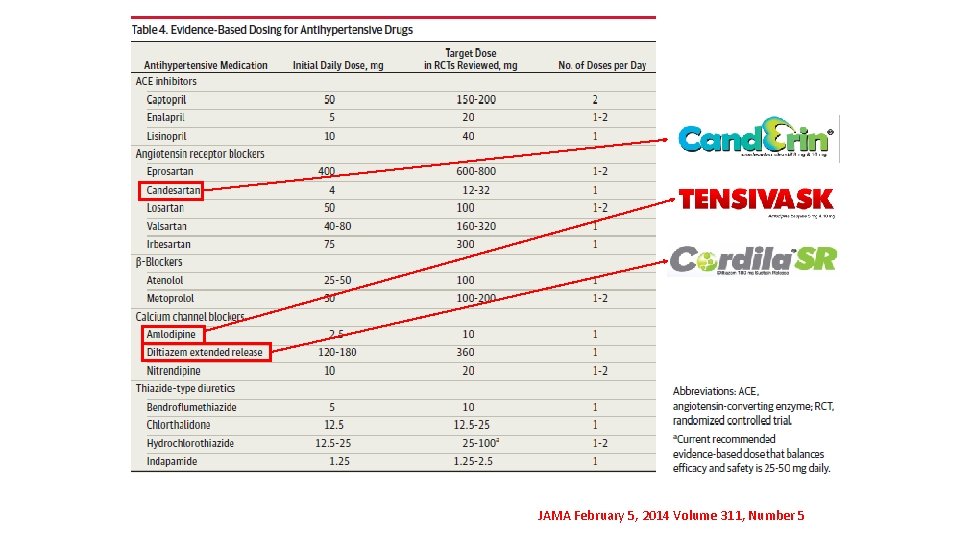
JAMA February 5, 2014 Volume 311, Number 5

BP LOWERING AGENT WHY ARB’S?
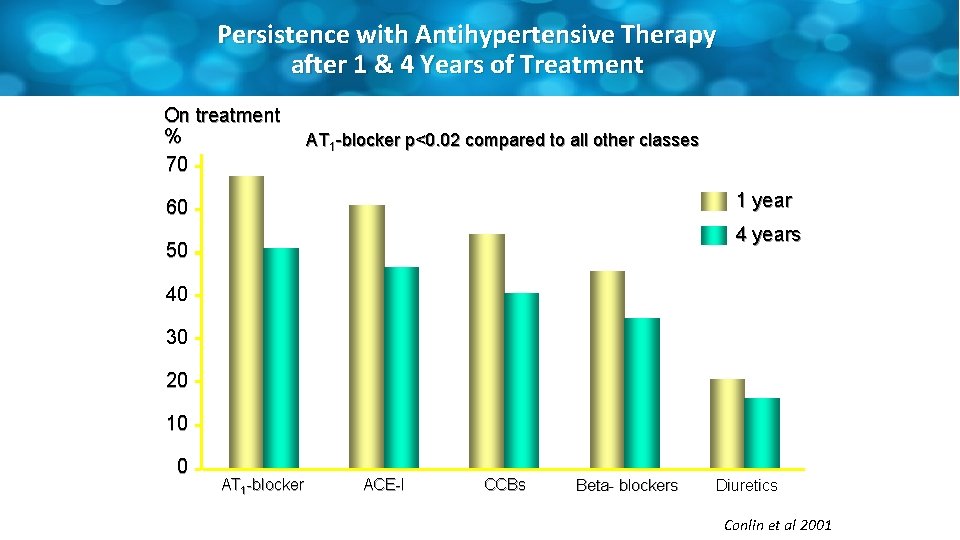
Persistence with Antihypertensive Therapy after 1 & 4 Years of Treatment On treatment % 70 AT 1 -blocker p<0. 02 compared to all other classes 1 year 60 4 years 50 40 30 20 10 0 AT 1 -blocker ACE-I CCBs Beta- blockers Diuretics Conlin et al 2001
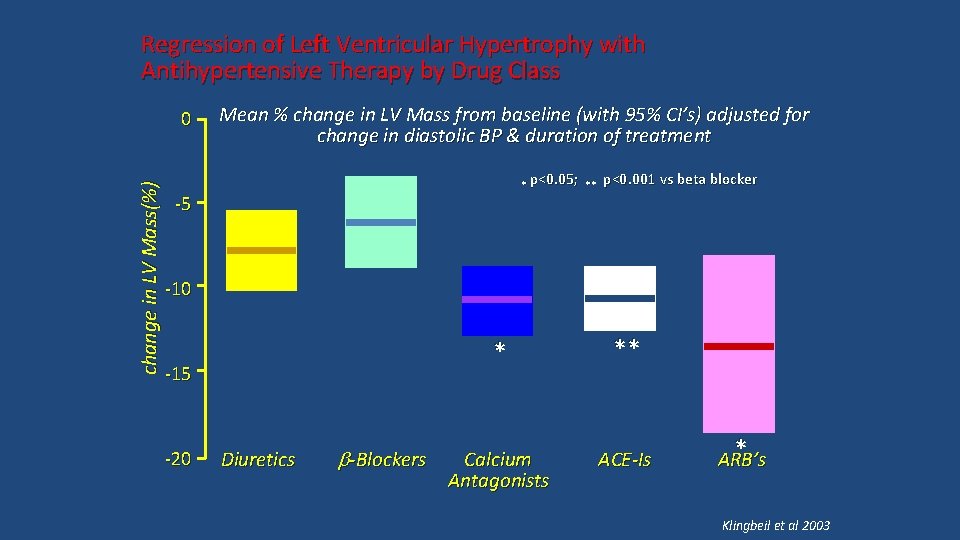
Regression of Left Ventricular Hypertrophy with Antihypertensive Therapy by Drug Class Mean % change in LV Mass from baseline (with 95% CI’s) adjusted for change in diastolic BP & duration of treatment * p<0. 05; ** -5 p<0. 001 vs beta blocker -10 * ** Calcium Antagonists ACE-Is -15 -20 Diuretics b-Blockers * change in LV Mass(%) 0 ARB’s Klingbeil et al 2003
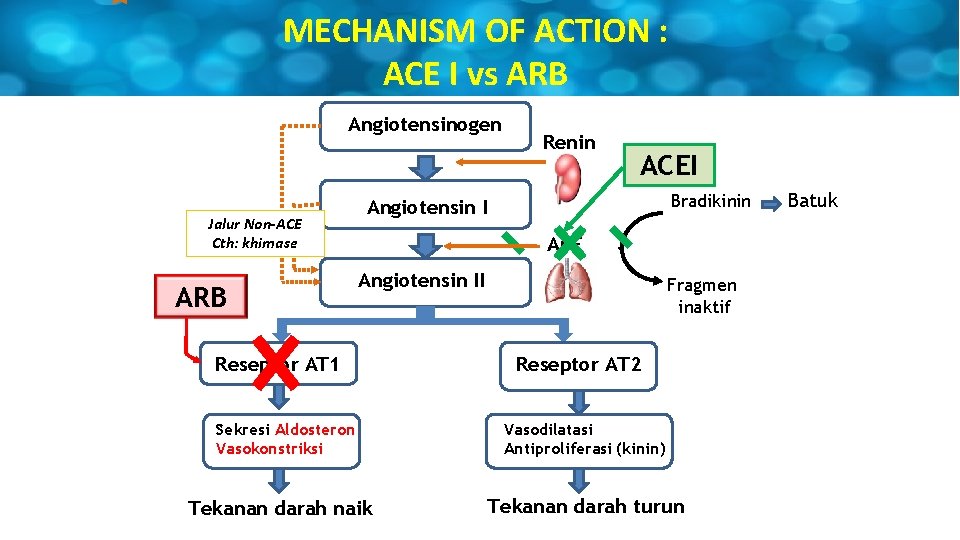
MECHANISM OF ACTION : ACE I vs ARB Angiotensinogen Jalur Non-ACE Cth: khimase ARB Renin ACEI Bradikinin Angiotensin I ACE Angiotensin II Fragmen inaktif Reseptor AT 1 Reseptor AT 2 Sekresi Aldosteron Vasokonstriksi Vasodilatasi Antiproliferasi (kinin) Tekanan darah naik Tekanan darah turun Batuk
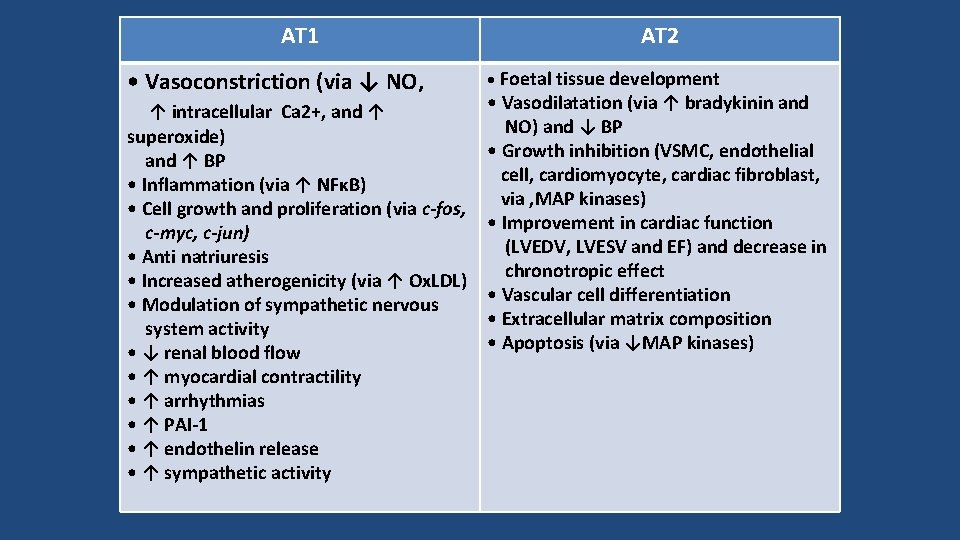
AT 1 • Vasoconstriction (via ↓ NO, ↑ intracellular Ca 2+, and ↑ superoxide) and ↑ BP • Inflammation (via ↑ NFκB) • Cell growth and proliferation (via c-fos, c-myc, c-jun) • Anti natriuresis • Increased atherogenicity (via ↑ Ox. LDL) • Modulation of sympathetic nervous system activity • ↓ renal blood flow • ↑ myocardial contractility • ↑ arrhythmias • ↑ PAI-1 • ↑ endothelin release • ↑ sympathetic activity AT 2 • Foetal tissue development • Vasodilatation (via ↑ bradykinin and NO) and ↓ BP • Growth inhibition (VSMC, endothelial cell, cardiomyocyte, cardiac fibroblast, via ‚MAP kinases) • Improvement in cardiac function (LVEDV, LVESV and EF) and decrease in chronotropic effect • Vascular cell differentiation • Extracellular matrix composition • Apoptosis (via ↓MAP kinases)

STRATEGIC CHOICE OF ARB’S The PARAMETER’s 1. 2. 3. 4. 5. 6. 7. GUIDELINES RECOMMENDATION AT 1 Affinity → Efficacy to reduce BP Protection on Target Organ Damage (TOD) Pleiotropic effect Safety Quality Assurance of Drugs Pharmacoeconomics
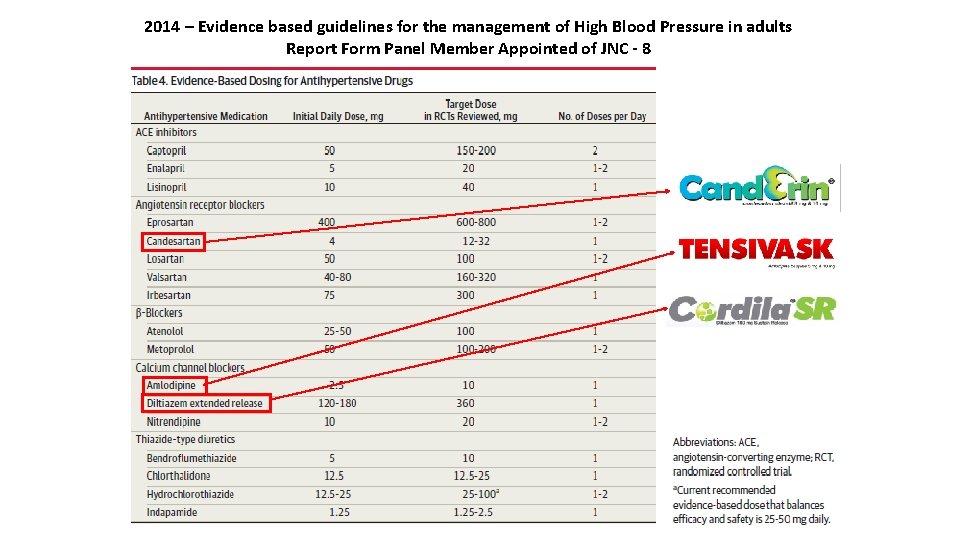
2014 – Evidence based guidelines for the management of High Blood Pressure in adults Report Form Panel Member Appointed of JNC - 8
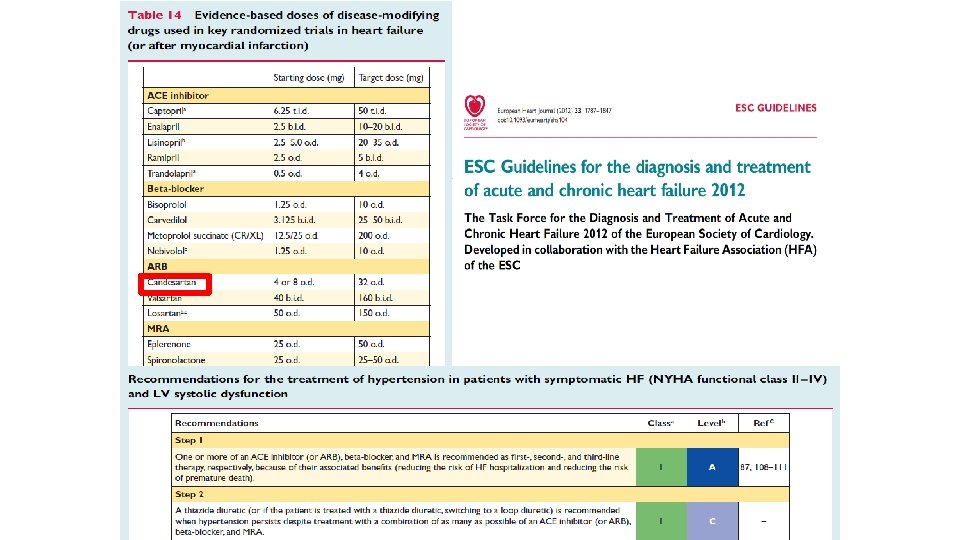

STRATEGIC CHOICE OF ARB’S The PARAMETER’s 1. 2. 3. 4. 5. 6. 7. GUIDELINES RECOMMENDATION AT 1 Affinity → Efficacy to reduce BP Protection on Target Organ Damage (TOD) Pleiotropic effect Safety Quality Assurance of Drugs Pharmacoeconomics
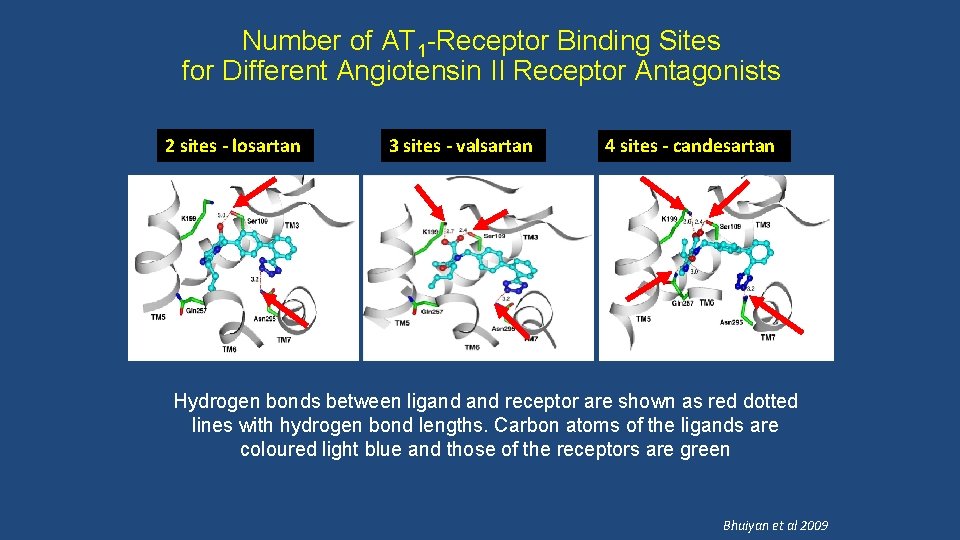
Number of AT 1 -Receptor Binding Sites for Different Angiotensin II Receptor Antagonists 2 sites - losartan 3 sites - valsartan 4 sites - candesartan Hydrogen bonds between ligand receptor are shown as red dotted lines with hydrogen bond lengths. Carbon atoms of the ligands are coloured light blue and those of the receptors are green Bhuiyan et al 2009
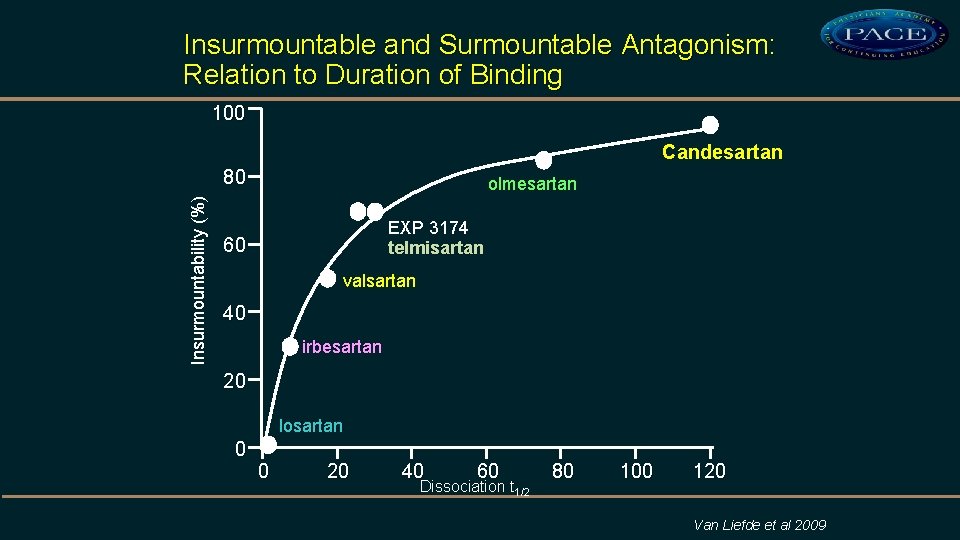
Insurmountable and Surmountable Antagonism: Relation to Duration of Binding 100 Candesartan Insurmountability (%) 80 olmesartan EXP 3174 telmisartan 60 valsartan 40 irbesartan 20 losartan 0 0 20 40 60 Dissociation t 1/2 80 100 120 Van Liefde et al 2009
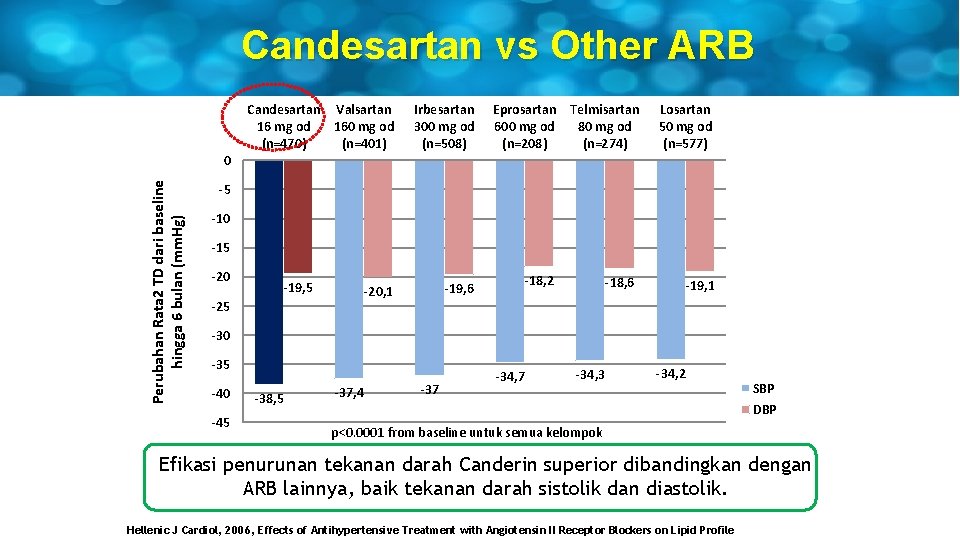
Candesartan vs Other ARB Candesartan Valsartan 16 mg od 160 mg od (n=470) (n=401) Irbesartan 300 mg od (n=508) Eprosartan Telmisartan 600 mg od 80 mg od (n=208) (n=274) Losartan 50 mg od (n=577) Perubahan Rata 2 TD dari baseline hingga 6 bulan (mm. Hg) 0 -5 -10 -15 -20 -19, 5 -25 -18, 2 -19, 6 -20, 1 -18, 6 -19, 1 -30 -35 -40 -45 -38, 5 -37, 4 -37 -34, 3 -34, 2 SBP DBP p<0. 0001 from baseline untuk semua kelompok Efikasi penurunan tekanan darah Canderin superior dibandingkan dengan ARB lainnya, baik tekanan darah sistolik dan diastolik. Hellenic J Cardiol, 2006, Effects of Antihypertensive Treatment with Angiotensin II Receptor Blockers on Lipid Profile
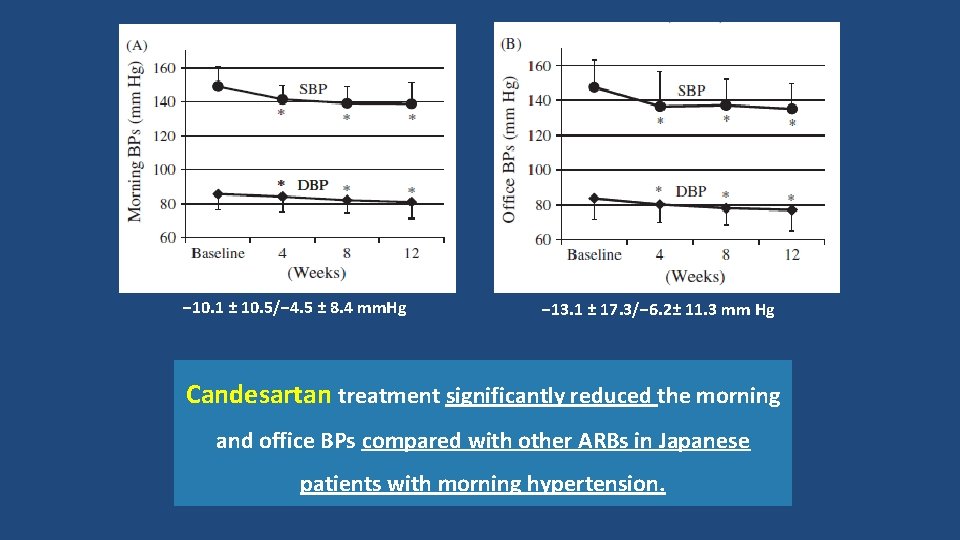
− 10. 1 ± 10. 5/− 4. 5 ± 8. 4 mm. Hg − 13. 1 ± 17. 3/− 6. 2± 11. 3 mm Hg Candesartan treatment significantly reduced the morning and office BPs compared with other ARBs in Japanese patients with morning hypertension.

STRATEGIC CHOICE OF ARB’S The PARAMETER’s 1. 2. 3. 4. 5. 6. 7. GUIDELINES RECOMMENDATION AT 1 Affinity → Efficacy to reduce BP Protection on Target Organ Damage (TOD) Pleiotropic effect Safety Quality Assurance of Drugs Pharmacoeconomics
Goals of Hypertension Treatment • In hypertensive patients, the primary goal of treatment is to achieve maximum reduction in the long-term total risk of cardiovascular disease • This requires treatment of the raised BP per se as well as of all associated reversible risk factors • BP should be reduces to at least below 140/90 mm. Hg (systolic/diastolic) and to lower values, if tolerated, in all hypertensive patients Mancia G, et al. 2007 ESH/ESC Guidelines for the Management of Arterial Hypertension. J Hypertens 2007; 25: 1105 -1187
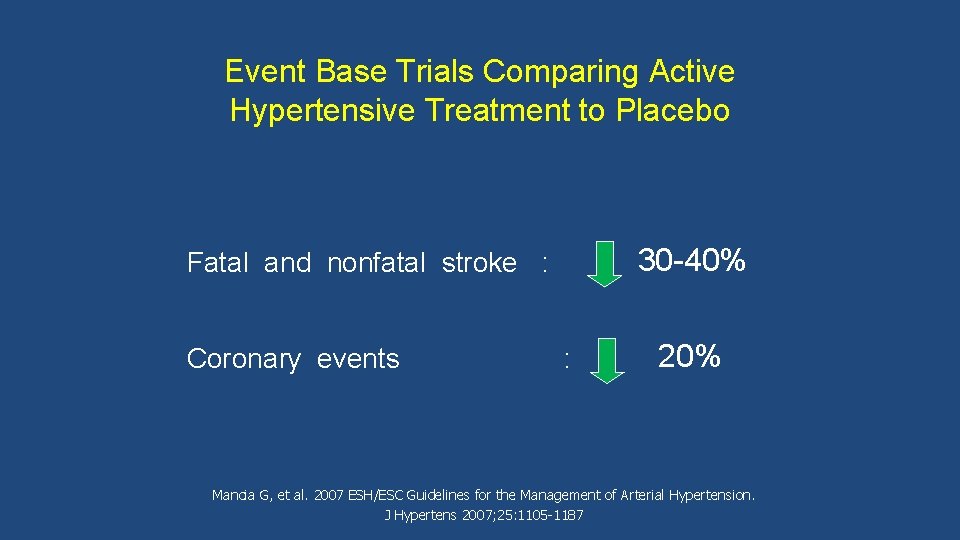
Event Base Trials Comparing Active Hypertensive Treatment to Placebo 30 -40% Fatal and nonfatal stroke : Coronary events : 20% Mancia G, et al. 2007 ESH/ESC Guidelines for the Management of Arterial Hypertension. J Hypertens 2007; 25: 1105 -1187
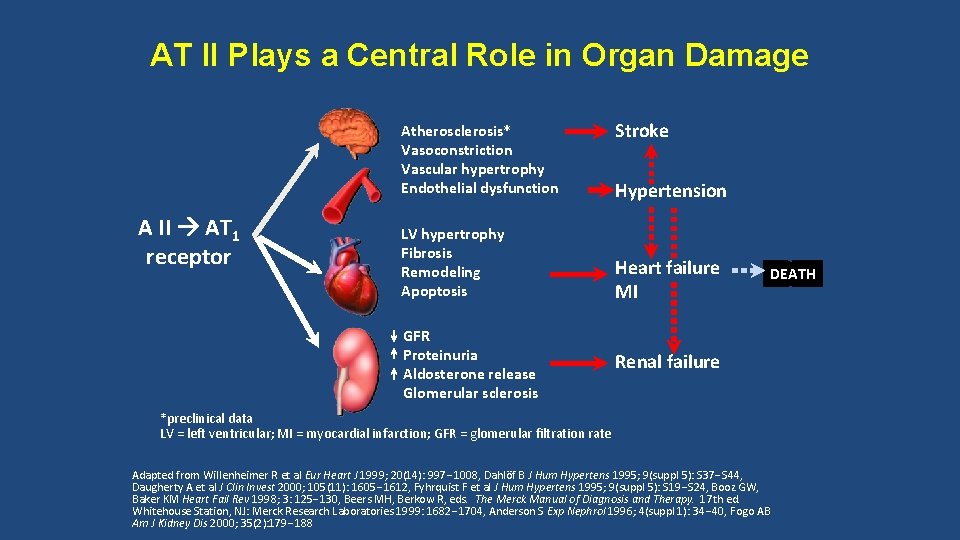
AT II Plays a Central Role in Organ Damage Atherosclerosis* Vasoconstriction Vascular hypertrophy Endothelial dysfunction A II AT 1 receptor LV hypertrophy Fibrosis Remodeling Apoptosis GFR Proteinuria Aldosterone release Glomerular sclerosis Stroke Hypertension Heart failure MI Renal failure *preclinical data LV = left ventricular; MI = myocardial infarction; GFR = glomerular filtration rate Adapted from Willenheimer R et al Eur Heart J 1999; 20(14): 997 1008, Dahlöf B J Hum Hypertens 1995; 9(suppl 5): S 37 S 44, Daugherty A et al J Clin Invest 2000; 105(11): 1605 1612, Fyhrquist F et al J Hum Hypertens 1995; 9(suppl 5): S 19 S 24, Booz GW, Baker KM Heart Fail Rev 1998; 3: 125 130, Beers MH, Berkow R, eds. The Merck Manual of Diagnosis and Therapy. 17 th ed. Whitehouse Station, NJ: Merck Research Laboratories 1999: 1682 1704, Anderson S Exp Nephrol 1996; 4(suppl 1): 34 40, Fogo AB Am J Kidney Dis 2000; 35(2): 179 188 DEATH
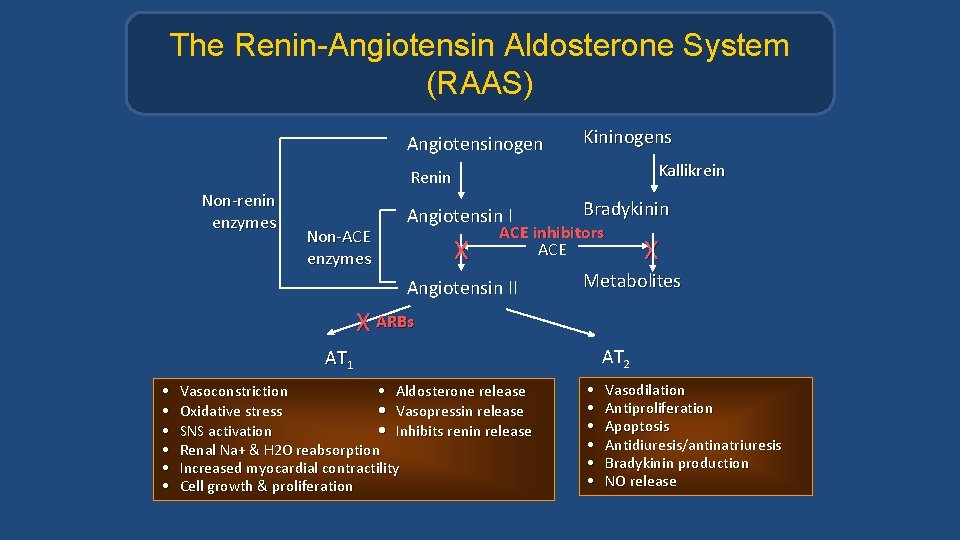
The Renin-Angiotensin Aldosterone System (RAAS) Angiotensinogen Kininogens Kallikrein Renin Non-renin enzymes Non-ACE enzymes Angiotensin I X Bradykinin ACE inhibitors ACE Angiotensin II X Metabolites X ARBs AT 2 AT 1 • • • Vasoconstriction • Aldosterone release Oxidative stress • Vasopressin release SNS activation • Inhibits renin release Renal Na+ & H 2 O reabsorption Increased myocardial contractility Cell growth & proliferation • • • Vasodilation Antiproliferation Apoptosis Antidiuresis/antinatriuresis Bradykinin production NO release
The Cardiovascular Continuum CANDESARTAN Clinical Study
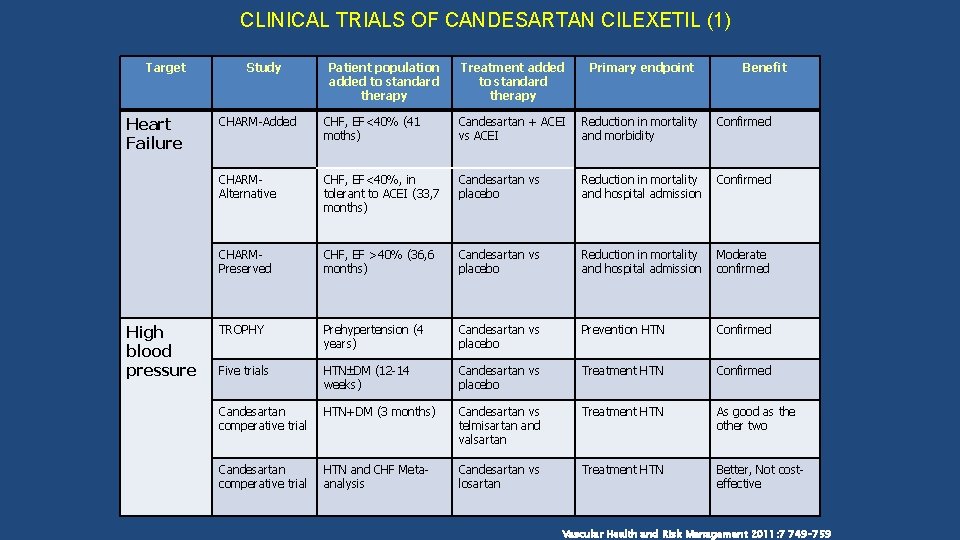
CLINICAL TRIALS OF CANDESARTAN CILEXETIL (1) Target Heart Failure High blood pressure Study Patient population added to standard therapy Treatment added to standard therapy Primary endpoint Benefit CHARM-Added CHF, EF<40% (41 moths) Candesartan + ACEI vs ACEI Reduction in mortality and morbidity Confirmed CHARMAlternative CHF, EF<40%, in tolerant to ACEI (33, 7 months) Candesartan vs placebo Reduction in mortality and hospital admission Confirmed CHARMPreserved CHF, EF >40% (36, 6 months) Candesartan vs placebo Reduction in mortality and hospital admission Moderate confirmed TROPHY Prehypertension (4 years) Candesartan vs placebo Prevention HTN Confirmed Five trials HTN DM (12 -14 weeks) Candesartan vs placebo Treatment HTN Confirmed Candesartan comperative trial HTN+DM (3 months) Candesartan vs telmisartan and valsartan Treatment HTN As good as the other two Candesartan comperative trial HTN and CHF Metaanalysis Candesartan vs losartan Treatment HTN Better, Not costeffective Vascular Health and Risk Management 2011: 7 749– 759
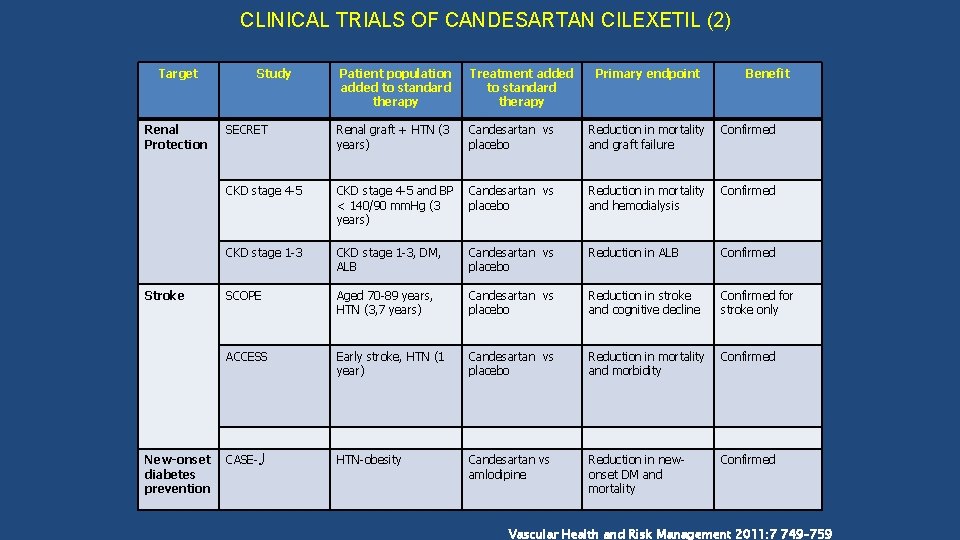
CLINICAL TRIALS OF CANDESARTAN CILEXETIL (2) Target Renal Protection Stroke New-onset diabetes prevention Study Patient population added to standard therapy Treatment added to standard therapy Primary endpoint Benefit SECRET Renal graft + HTN (3 years) Candesartan vs placebo Reduction in mortality and graft failure Confirmed CKD stage 4 -5 and BP < 140/90 mm. Hg (3 years) Candesartan vs placebo Reduction in mortality and hemodialysis Confirmed CKD stage 1 -3, DM, ALB Candesartan vs placebo Reduction in ALB Confirmed SCOPE Aged 70 -89 years, HTN (3, 7 years) Candesartan vs placebo Reduction in stroke and cognitive decline Confirmed for stroke only ACCESS Early stroke, HTN (1 year) Candesartan vs placebo Reduction in mortality and morbidity Confirmed CASE- HTN-obesity Candesartan vs amlodipine Reduction in newonset DM and mortality Confirmed Vascular Health and Risk Management 2011: 7 749– 759
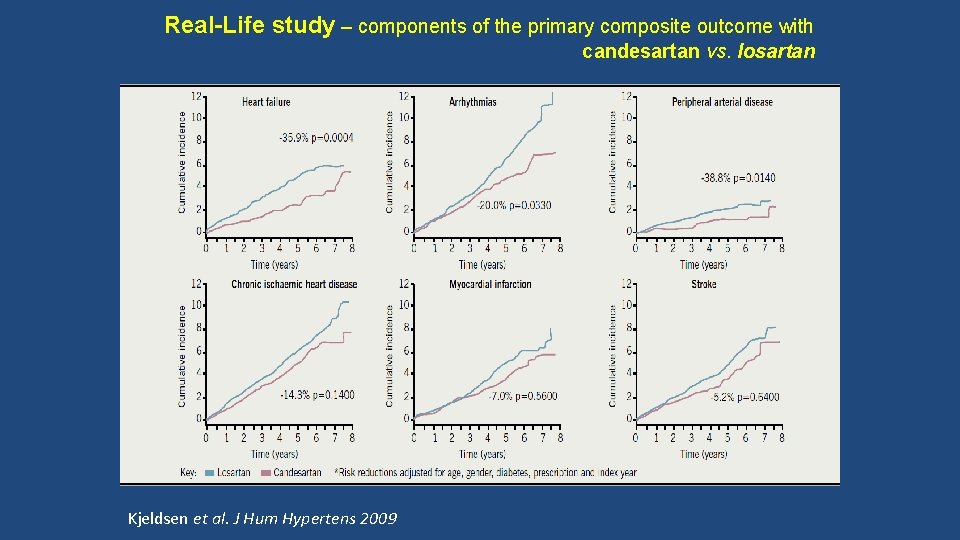
Real-Life study – components of the primary composite outcome with candesartan vs. losartan Kjeldsen et al. J Hum Hypertens 2009
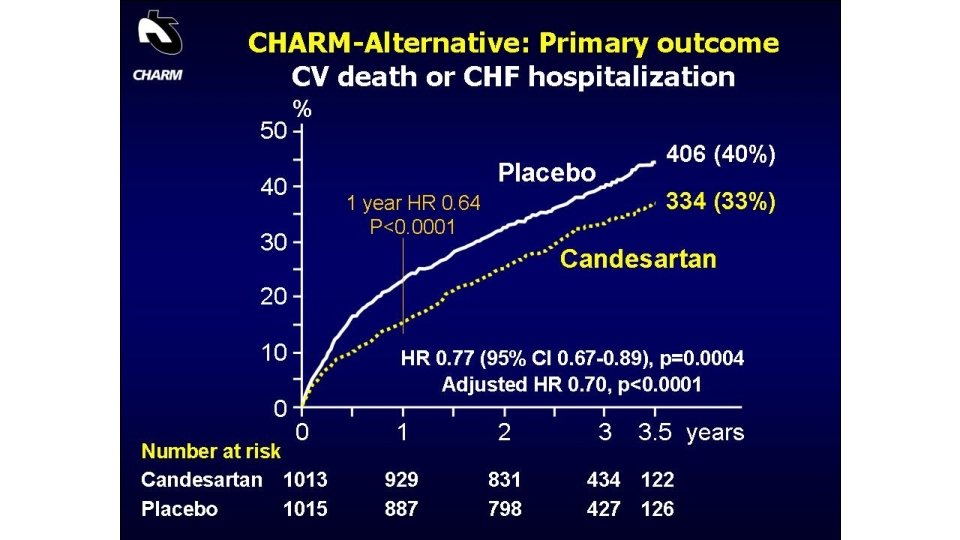
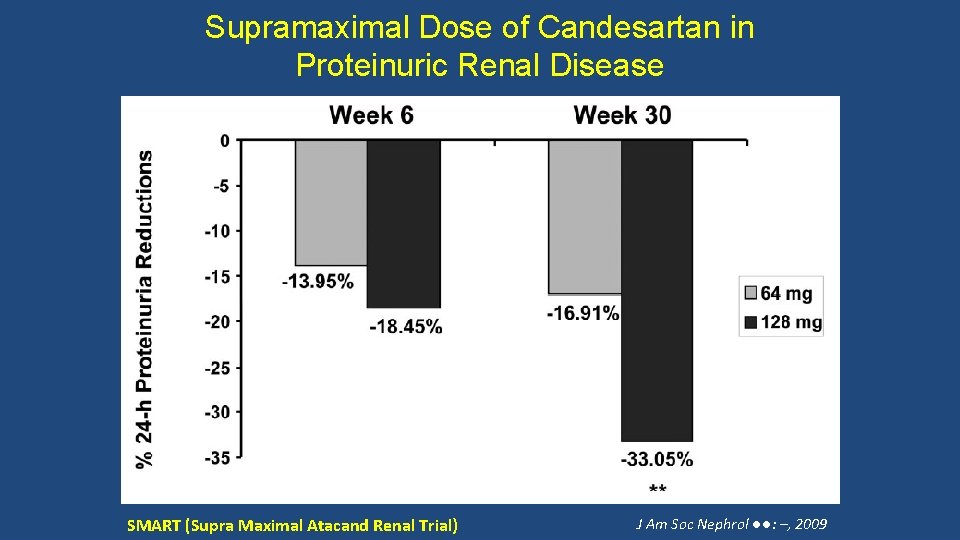
Supramaximal Dose of Candesartan in Proteinuric Renal Disease SMART (Supra Maximal Atacand Renal Trial) J Am Soc Nephrol ●●: –, 2009

STRATEGIC CHOICE OF ARB’S The PARAMETER’s 1. 2. 3. 4. 5. 6. 7. GUIDELINES RECOMMENDATION AT 1 Affinity → Efficacy to reduce BP Protection on Target Organ Damage (TOD) Pleiotropic effect Safety Quality Assurance of Drugs Pharmacoeconomics
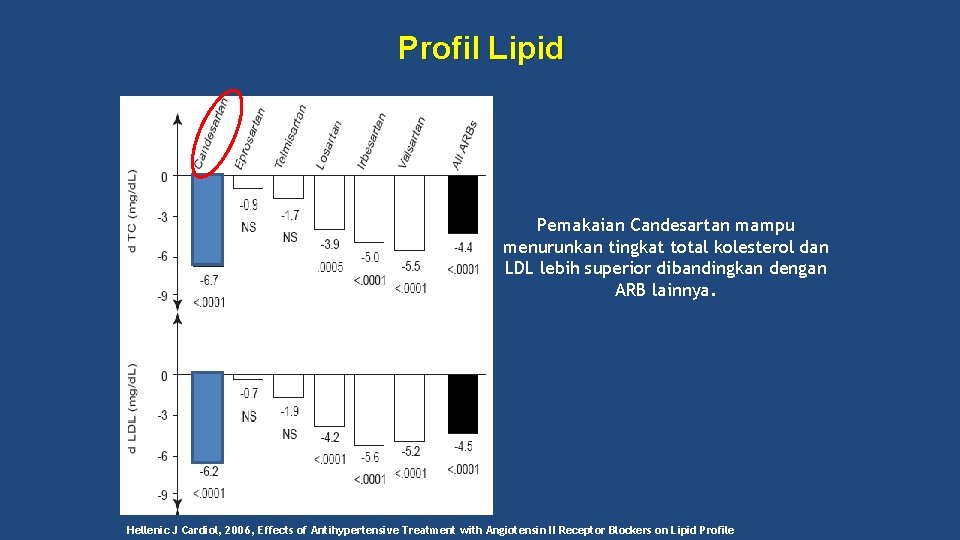
Profil Lipid Pemakaian Candesartan mampu menurunkan tingkat total kolesterol dan LDL lebih superior dibandingkan dengan ARB lainnya. Hellenic J Cardiol, 2006, Effects of Antihypertensive Treatment with Angiotensin II Receptor Blockers on Lipid Profile
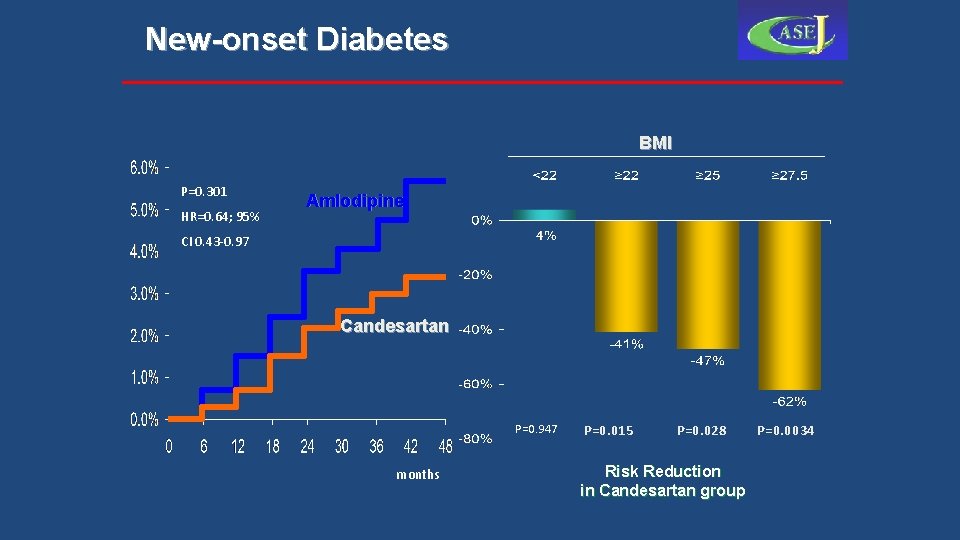
New-onset Diabetes BMI P=0. 301 HR=0. 64; 95% Amlodipine CI 0. 43 -0. 97 Candesartan P=0. 947 months P=0. 015 P=0. 028 Risk Reduction in Candesartan group P=0. 0034

STRATEGIC CHOICE OF ARB’S The PARAMETER’s 1. 2. 3. 4. 5. 6. 7. GUIDELINES RECOMMENDATION AT 1 Affinity → Efficacy to reduce BP Protection on Target Organ Damage (TOD) Pleiotropic effect Safety Quality Assurance of Drugs Pharmacoeconomics

STRATEGIC CHOICE OF ARB’S The PARAMETER’s 1. 2. 3. 4. 5. 6. 7. GUIDELINES RECOMMENDATION AT 1 Affinity → Efficacy to reduce BP Protection on Target Organ Damage (TOD) Pleiotropic effect Safety Quality Assurance of Drugs Pharmacoeconomics

Bioequivalence study of CANDERIN®
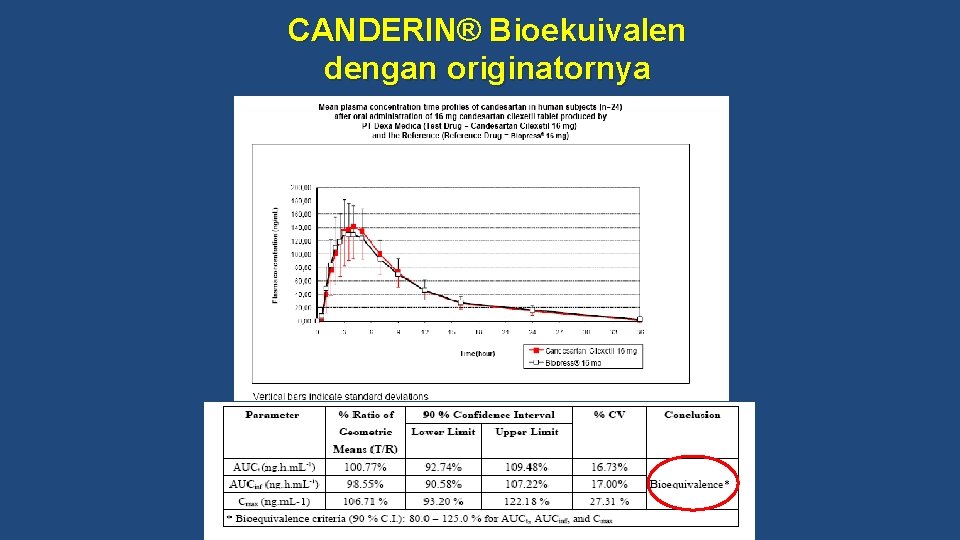
CANDERIN® Bioekuivalen dengan originatornya

STRATEGIC CHOICE OF ARB’S The PARAMETER’s 1. 2. 3. 4. 5. 6. 7. GUIDELINES RECOMMENDATION AT 1 Affinity → Efficacy to reduce BP Protection on Target Organ Damage (TOD) Pleiotropic effect Safety Quality Assurance of Drugs Pharmacoeconomics

WHY COPY PRODUCTS EXIST? • Original products loose it’s patent protection • Copy Products have more opportunities – Definition: Branded copy products & OGB – Health Care Cost is continue to increase, needs medicines with lower price, especially for chronic disease. – Stringent GMP and BIOEQUIVALENCY requirements ensure the Quality of the products, compare to its originator

Conclusions • Despite the idea that all ARBs are the same and show a ‘class’ effect, significant pharmacological differences with respect to efficacy and duration of action are apparent within the ARB class. • Such differences may translate into differences in BP reduction, and thus a reduction in CV risk. These differences should be taken into account by the prescribing physician • Long-acting ARB Candesartan have better CV outcomes. • Copy product may reduces health care cost, but must be bioequivalent compare to originator (Quality Assurance of Drugs)

TERIMA KASIH

New WHO Report, April 2011, highlights NCD and CVD Deaths worldwide ! n New WHO report reveals an ‘impending disaster’ caused by a rise in deaths from heart disease and other non-communicable diseases n Geneva, April 27, 2011 – The World Health Organization has today published a report on non-communicable diseases (NCDs), including cardiovascular disease (CVD) which is the number one killer worldwide, and has described them as an ‘impending disaster’ for health, society and national economies. n The World Heart Federation welcomed report recommendations for a ‘forceful response’ to the potential tragedy posed by noncommunicable diseases, particularly in the developing world
7. 6 Million deaths each year attributable to suboptimal blood pressure n 7. 6 million deaths each year (13. 5% of total) are attributable to high blood pressure. n 54% of Stroke and 47% of coronary heart disease worldwide attributed to high blood pressure. n Data obtained from Global Burden of Disease study, updated in 2008. Lawes, Hoorn, Rodgers, for ISH: Lancet 2008; 371: 1513 -18

- Slides: 49