Story Game Directions Junior Chemist Game Preparation Game

































- Slides: 43

Story Game Directions Junior Chemist! Game Preparation Game Pieces Play the Game Objectives Credits Copyright Notice *By: Duaa Saleh*

The Story of “Junior Chemist” Dr. Newtonstein is a mad scientist who is working on a new formula that is sure to cure this virus that has been going around. He just needs a few more ingredients to put the final touch on his formula and strike it rich! Since he cannot leave the formula unattended, he needs your help in getting those key ingredients. He agrees to share the money and fame he will get from this formula with the person who finds those six ingredients! You must now compete with other junior scientists to help Dr. Newtonstein. There are five containers of each ingredient throughout the Science Research Building. Since all ingredients are under lock and key, you need to answer questions to unlock the locker for each ingredient—in just one minute per question! You will need to collect a red, blue, yellow, purple, green, and orange bottle and bring them all to Dr. Newtonstein before someone else beats you to it! Now’s your chance, Junior Chemist! Solve some problems, name some compounds, and label some formulas! Answer a variety of Chemistry questions and be the first to collect each of the six ingredients so you can become a famous scientist with Dr. Newtonstein and strike it rich!! Home Game Directions

Game Directions The goal of the game is to help Dr. Newtonstein with his anti-viral formula by earning the six colors/ingredients so you can become a famous Junior Chemist and make some money for it! To play the game, you need at least two players but five players calls for good competition! You have to answer a question of each type. The questions are categorized by colors: Red = Scientific Notation problems Blue = Conversion problems Yellow = Ionic Compounds questions Green = Molecular Compounds questions Orange = Electron Configuration questions Purple = Acids questions Each player takes a turn drawing a wedge out an envelope. Players choose which envelope they want to draw from but once they correctly answer a question from that envelope, they may not draw from it again (this means they have earned this ingredient). Each player gets one minute to answer a question. Players are not to see other players’ answer selections. If a player answers the question incorrectly, he/she is to put the wedge back into the envelope. No calculators may be used! To win the game you have to be the first one to collect all six colors/ingredients! Home Game Preparation Play the Game

Game Preparation Materials: *Printout of slides 5 & 6 *Scissors to cut out game pieces *Six envelopes *Stopwatch/Timer *Scrap paper (optional) Preparation: You will need to print and cut out the game pieces from slides 5 & 6. You will need six envelopes (one for each color/ingredient). Place the wedges in the six envelopes and label them with the color name (or ingredient name). Home Game Pieces

Game Pieces 7 1 19 13 8 2 20 14 9 3 25 26 21 15 27 More…

Game Pieces 10 4 22 16 11 5 23 17 12 6 28 29 24 18 30 Home

Question Board 19 7 Draw a wedge piece and click on the question number you draw! 1 13 20 8 2 14 3 15 4 16 5 28 23 11 17 29 24 12 6 27 22 10 Home 26 21 9 Game Directions 25 18 30

Question 1 Your lab manual tells you that you will need a substance with identification number 3, 400, 000 for your lab. All the substances however are labeled in scientific notation. Which label applies to the substance you need? a. 3. 4 x 10 -6 b. 3. 4 x 106 c. 3. 4 x 10 -5 d. 3. 4 x 105 e. 34 x 105

Question 2 You need 157 n. L of a chemical to make a formula, but your graduated cylinder only measures in m. L. How many m. L of the chemical do you need? a. 1. 57 x 10 -4 b. 1. 57 x 104 c. 1. 57 x 10 -8 d. 1. 57 x 108 e. 1. 57 x 10 -6

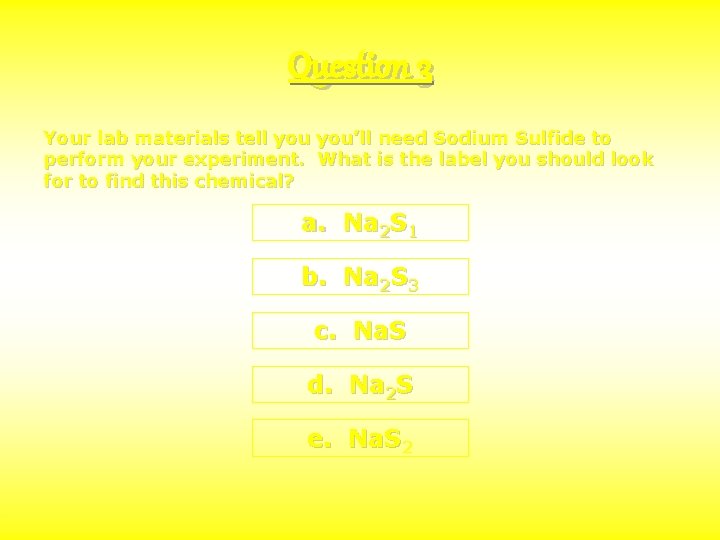
Question 3 Your lab materials tell you’ll need Sodium Sulfide to perform your experiment. What is the label you should look for to find this chemical? a. Na 2 S 1 b. Na 2 S 3 c. Na. S d. Na 2 S e. Na. S 2

Question 4 Your lab partner who is not familiar with chemical formulas brings a group of chemicals to your lab station but they do not have formulas on the label. The first chemical you’ll need is Cl 2 O 8. Which chemical are you looking for? a. Dichlorine Hexoxide b. Trichlorine Heptoxide c. Dichlorine Heptoxide d. Trichlorine Octoxide e. Dichlorine Octoxide

Question 5 You are trying to build an atomic model for Neon. In order to create and set up the models correctly, you need to know the electron configuration of Neon (atomic number 10). What would your electron configuration be? a. 1 s 22 s 42 p 4 b. 1 s 21 p 22 s 22 p 4 c. 1 s 22 p 6 d. 1 s 22 s 42 p 4 e. 1 s 22 p 43 s 2

Question 6 You see an acid with the label HNO 2 on it. What is the name of this acid? a. Nitrous Acid b. Nitric Acid c. Hydronitric Acid d. Hydronitrous Acid e. Hyponitric Acid

Question 7 Your lab partner gets confused with numbers that have a lot of zeros. So when you tell him to write down 0. 000245, he kindly asks you if there’s an easier way to write it. How would you express this number to him in Scientific Notation? a. 2. 45 x 103 b. 2. 45 x 10 -3 c. 2. 45 x 104 d. 2. 45 x 10 -4 e. 245 x 10 -6

Question 8 You measured the mass of your precipitate after filtration and found it to be 0. 065 kg. However, your measurement needs to be given in dg. What would you record your measurement as in dg? a. 6. 5 x 10 -7 b. 6. 5 x 107 c. 6. 5 x 103 d. 6. 5 x 10 -3 e. 6. 5 x 105

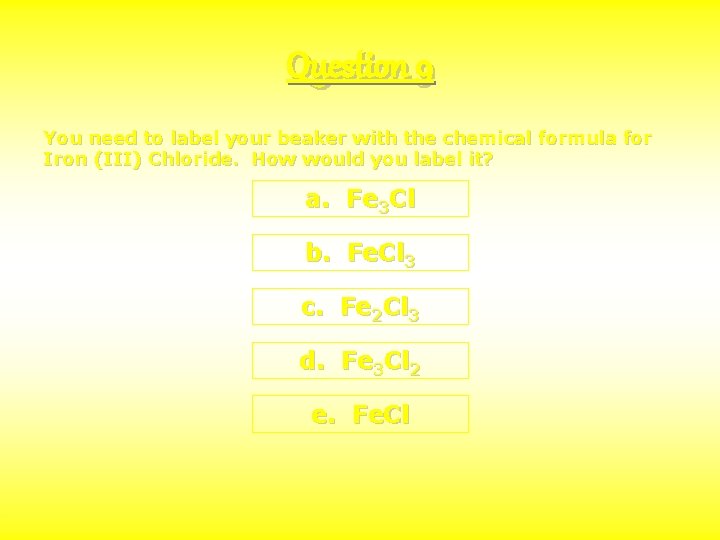
Question 9 You need to label your beaker with the chemical formula for Iron (III) Chloride. How would you label it? a. Fe 3 Cl b. Fe. Cl 3 c. Fe 2 Cl 3 d. Fe 3 Cl 2 e. Fe. Cl

Question 10 There is a confusion in the lab about the proper name for N 3 H 4, a chemical needed to perform an experiment Your help is needed so that people don’t use the wrong chemical in their lab! Which chemical should people be using? a. Trinitrogen Tetrahydride b. Trinitrogen Pentahydride c. Dinitrogen Tetrahydride d. Dinitrogen Trihydride e. Tetranitrogen Trihydride

Question 11 You are trying to explain to someone how the electrons spin in their orbitals for an Oxygen atom. You need to know the electron configuration in order to know how many orbitals there and how many electrons. The only way you can do this is by expressing the electron configuration of Oxygen (atomic number 8). What is Oxygen’s electron configuration? a. 1 s 22 p 6 b. 1 s 21 p 22 s 22 p 2 c. 1 s 22 p 6 d. 1 s 22 s 42 p 2 e. 1 s 22 p 4

Question 12 You see an acid with the label HF on it. What is the name of this acid? a. Fluorous Acid b. Fluoric Acid c. Hydrofluoric Acid d. Hydrofluorous Acid e. Hypofluourous Acid

Question 13 You have to record over a hundred numbers on your lab sheet but you don’t have enough room (especially with all those numbers that will have plenty of zeros!). Your first data value is 201, 000. How would you write it in Scientific Notation? a. 2. 01 x 105 b. 2. 01 x 10 -5 c. 2. 01 x 103 d. 2. 01 x 10 -3 e. 201 x 103

Question 14 The vapor from your chemical formula traveled a distance of 642 cm. How many km has the vapor traveled? a. 6. 42 x 10 -5 b. 6. 42 x 107 c. 6. 42 x 10 -7 d. 6. 42 x 103 e. 6. 42 x 10 -3

Question 15 You need some Magnesium Nitride to complete your experiment but you see so many formulas that look alike! What is the correct chemical formula you should look for? a. Mg. N 3 b. Mg 2 N 3 c. Mg 3 N 2 d. Mg 2 N e. Mg. N 2

Question 16 You have a test tube labeled P 2 O 3. Which chemical should you have in that test tube? a. Diphosphorus Hexoxide b. Diphosphorus Trioxide c. Diphosphorus Tetroxide d. Triphosphorus Dioxide e. Triphosphorus Tetroxide

Question 17 Your science fair project involves you creating a spinning model of the Sodium atom. For the heading of your model, you need to put the electron configuration of Sodium (atomic number 11) so that observers understand why you have a certain number of electrons and orbitals. What should the heading on your Sodium atomic model be? a. 1 s 22 p 63 p 1 b. 1 s 21 p 62 s 2 p 1 c. 1 s 22 p 7 d. 1 s 22 p 63 s 1 e. 1 s 22 p 43 s 23 p 1

Question 18 You see an acid with the label H 2 SO 4 on it. What is the name of this acid? a. Sulfurous Acid b. Hyposulfuric Acid c. Hydrosulfuric Acid d. Hydrosulfurous Acid e. Sulfuric Acid

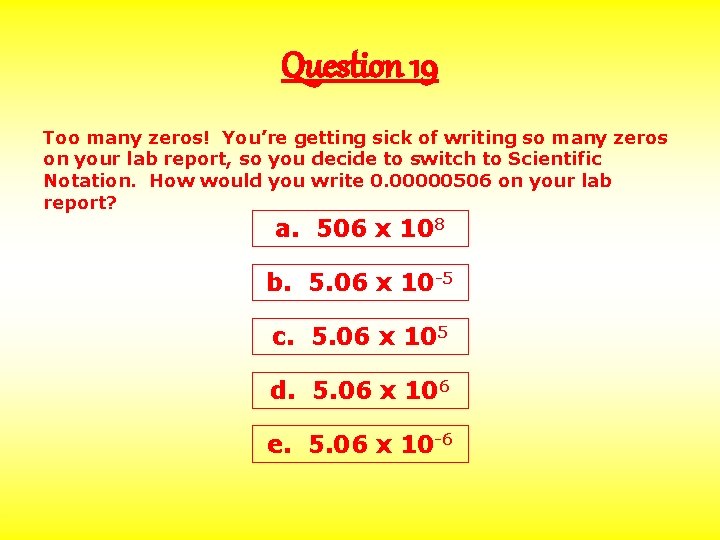
Question 19 Too many zeros! You’re getting sick of writing so many zeros on your lab report, so you decide to switch to Scientific Notation. How would you write 0. 00000506 on your lab report? a. 506 x 108 b. 5. 06 x 10 -5 c. 5. 06 x 105 d. 5. 06 x 106 e. 5. 06 x 10 -6

Question 20 You need to add 1. 44 m. L of water to your chemical formula. You’ve been doing all your measurements in m. L. How many m. L of water should you add? a. 1. 44 x 10 -3 b. 1. 44 x 103 c. 1. 44 x 10 -9 d. 1. 44 x 109 e. 1. 44 x 10 -4

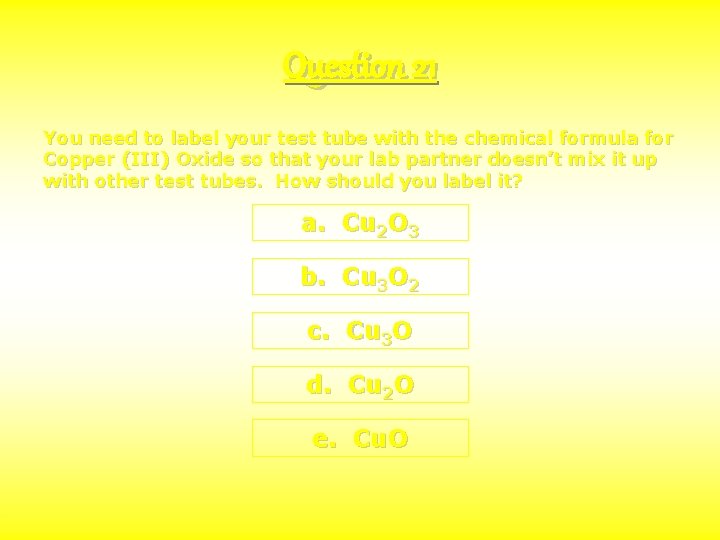
Question 21 You need to label your test tube with the chemical formula for Copper (III) Oxide so that your lab partner doesn’t mix it up with other test tubes. How should you label it? a. Cu 2 O 3 b. Cu 3 O 2 c. Cu 3 O d. Cu 2 O e. Cu. O

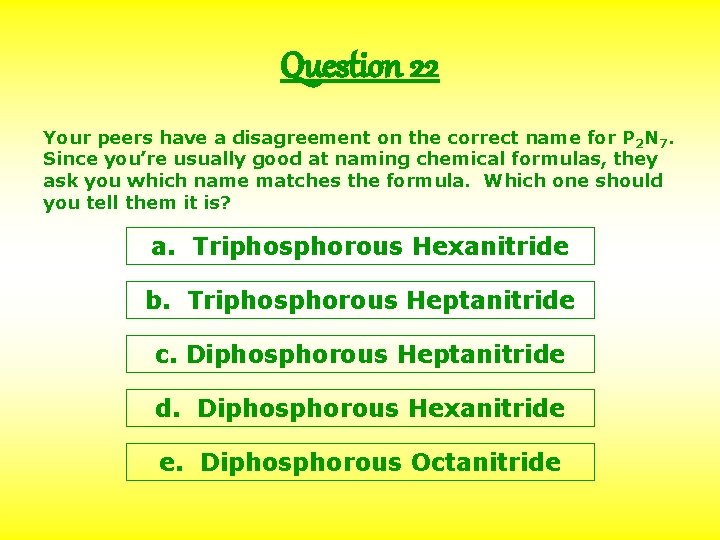
Question 22 Your peers have a disagreement on the correct name for P 2 N 7. Since you’re usually good at naming chemical formulas, they ask you which name matches the formula. Which one should you tell them it is? a. Triphosphorous Hexanitride b. Triphosphorous Heptanitride c. Diphosphorous Heptanitride d. Diphosphorous Hexanitride e. Diphosphorous Octanitride

Question 23 Your friend needs help figuring out the electron configuration for Phosphorus for his homework. You explain to him how to find electron configuration and show him what the final answer should be. How should the electron configuration of Phosphorus (atomic number 15) be expressed? a. 1 s 22 p 63 p 5 b. 1 s 22 p 63 s 23 p 3 c. 1 s 22 p 63 p 5 d. 1 s 22 p 43 s 23 p 44 s 1 e. 1 s 22 p 43 s 23 p 5

Question 24 You see an acid with the label HCl on it. What is the name of this acid? a. Chlorous Acid b. Chloric Acid c. Hydrochlorous Acid d. Hydrochloric Acid e. Hypochloric Acid

Question 25 Your friend argues with you that you cannot write 6, 402 in Scientific Notation because it does not have a lot of zeros. You explain to him that Scientific Notation doesn’t always deal with zeros and show him how to convert the number from standard form. How should you express 6, 402 be in Scientific Notation? a. 64. 02 x 103 b. 6. 402 x 10 -3 c. 6. 402 x 103 d. 6. 402 x 10 -4 e. 6. 402 x 104

Question 26 You need to measure 5, 000 dg of a substance, but your balance beam only measures in Mg. How many Mg of the substance should you weigh? a. 5 x 10 -7 b. 5 x 1010 c. 5 x 10 -10 d. 5 x 10 -4 e. 5 x 104

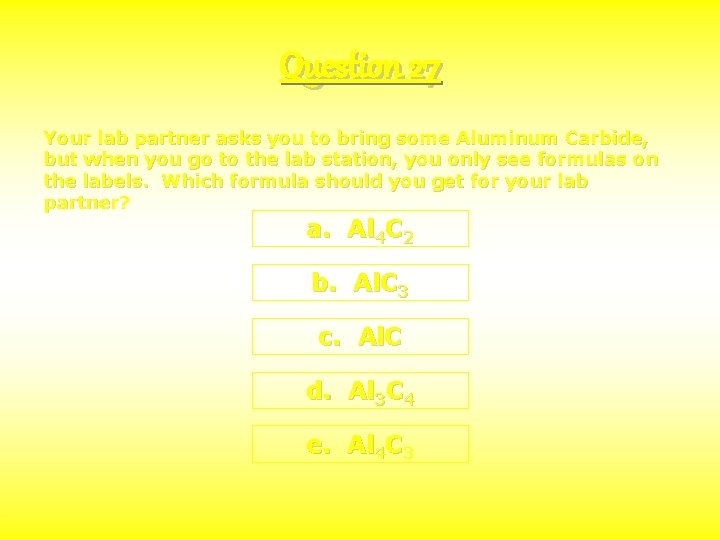
Question 27 Your lab partner asks you to bring some Aluminum Carbide, but when you go to the lab station, you only see formulas on the labels. Which formula should you get for your lab partner? a. Al 4 C 2 b. Al. C 3 c. Al. C d. Al 3 C 4 e. Al 4 C 3

Question 28 Your teacher explains to you that Carbon and Oxygen can mix to form various gases. She asks you to do some research on C 2 O. Since it’s easier to research a name rather than a formula, what should you look for information on? a. Dicarbon Dioxide b. Monocarbon Dioxide c. Monocarbon Oxide d. Dicarbon Monoxide e. Carbon Dioxide

Question 29 You need to draw the arrangement of the Fluorine atom as part of your lab write up. You’ve made mistakes and had to start over because you realize you can’t draw the atomic model without knowing the electron configuration! What is the electron configuration of Fluorine (atomic number 9)? a. 1 s 22 p 5 b. 1 s 22 p 43 s 1 c. 1 s 22 p 43 p 1 d. 1 s 21 p 22 s 22 p 3 e. 1 s 22 s 23 p 3

Question 30 You see an acid with the label HBr. O 2 on it. What is the name of this acid? a. Bromic Acid b. Bromous Acid c. Hydrobromic Acid d. Hydrobromous Acid e. Hypobromic Acid

Correct! You get to keep your wedge piece! Next player’s turn! Back to Questions A Message to the Game Winner…

Incorrect! Return your wedge piece! Next player’s turn! Back to Questions

CONGRATULATIONS! Hey, Junior Chemist! You’ve just helped Dr. Newtonstein complete his anti-viral formula!!! Your name will be published in the magazine, and you will receive a nice monetary reward of Congratulations! Tape your color wheel together, put your name on it, and pin it up on the classroom bulletin board! ***GREAT JOB!!!*** Home

Educational Objectives • Audience Chemistry (Grades 9 -12) • Subject Area Objectives Chemistry (General) – Students will be able to convert numbers in standard form to numbers in scientific notation. – Students will convert to and from various units in the metric system. – Students will be able to write formulas for ionic compounds. – Students will be able to name molecular compounds. – Students will be able to give the electron configuration of various elements. – Students will be able to name acids when given their formulas. Home

Credits All teachers and students at non-profit schools can use, revise, or adapt this game at will at no cost on the condition that all prior designers are cited. • Originally designed by Duaa Saleh, Wayne State University, October 6, 2007 “Junior Chemist. ” Home

Copyright Notice • © Copyright 2007 Duaa Saleh • Permission to copy this game at no cost is granted to all teachers and students of non-profit schools. • Permission is also granted to all teachers and students of non-profit schools to make revisions to this game for their own purposes, on the condition that this copyright page and the credits page remain part of the game. Teachers and students who adapt the game should add their names and affiliations to the credits page without deleting any names already there. Home