Storage and Management of Vaccines Neelam Ali Senior




















- Slides: 24

Storage and Management of Vaccines Neelam Ali Senior Community Services Pharmacist

The Cold. Chain • Maintaining vaccines within the manufacturer’s recommended storage temperature during transport and storage until the point of administration


Why is the “Cold chain” so important? • Efficacy depends on correct storage o o conditions +2 C to + 8 C • Compliance with Specific Product Characteristics and marketing authorisation • Assurance and confidence in a potent product • Ensuring maximum benefit from immunisation

Storage of vaccines outside recommended storage temperatures can lead to: • Deterioration in the vaccine and failure to produce a satisfactory level of immunity – Heat speeds up decline in potency - ↓shelf life – Freezing causes • Increased reactogenicity & loss of potency • can lead to hair line cracks in ampoules, vials or prefilled syringes causing contamination of contents

Vaccine Stability Temperature Sensitivity • Sensitive to Cold and Heat Light Sensitivity • Sensitive to strong light, sunlight, ultraviolet and fluorescent light (neon) • All vaccines should be stored in their original packaging until they are administered

Storage and Management of Vaccines • Receipt and Transport • Storage • Temperature monitoring • Use in vaccination sessions • Disposal and spillage • Disruption of the cold chain

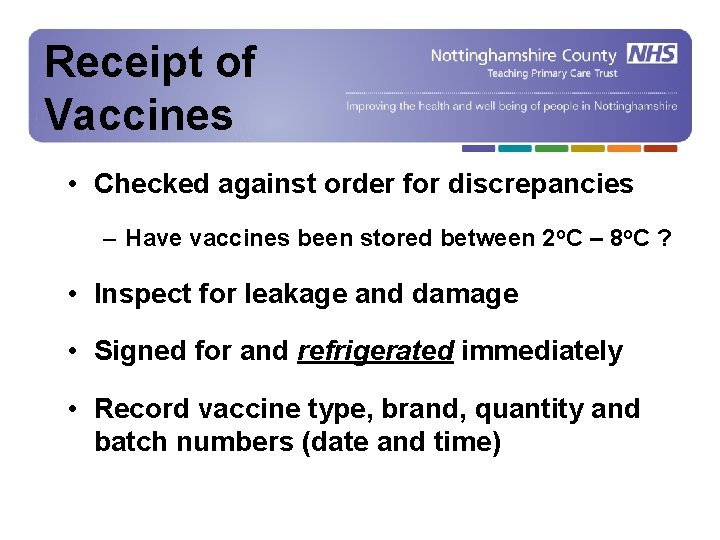
Receipt of Receipt Vaccines of Vaccines • Checked against order for discrepancies – Have vaccines been stored between 2 o. C – 8 o. C ? • Inspect for leakage and damage • Signed for and refrigerated immediately • Record vaccine type, brand, quantity and batch numbers (date and time)

Transport of Vaccines • Insulated validated cool boxes • Cool boxes – Fridge packs – Frozen packs • Spaces in cool box filled with insulating material • Vaccines should not be in direct contact with cool packs

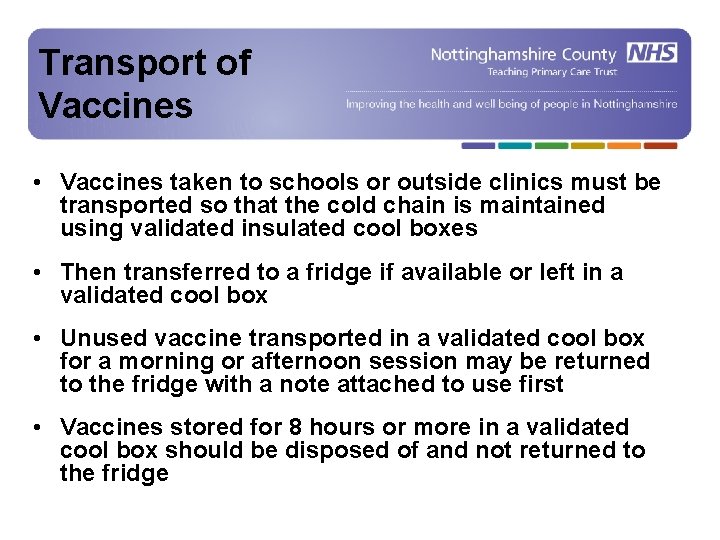
Transport of Vaccines • Vaccines taken to schools or outside clinics must be transported so that the cold chain is maintained using validated insulated cool boxes • Then transferred to a fridge if available or left in a validated cool box • Unused vaccine transported in a validated cool box for a morning or afternoon session may be returned to the fridge with a note attached to use first • Vaccines stored for 8 hours or more in a validated cool box should be disposed of and not returned to the fridge

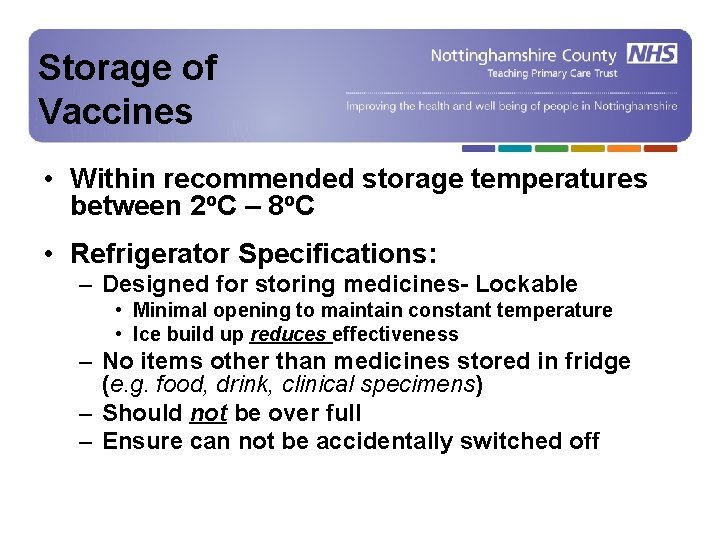
Storage of Vaccines • Within recommended storage temperatures between 2 o. C – 8 o. C • Refrigerator Specifications: – Designed for storing medicines- Lockable • Minimal opening to maintain constant temperature • Ice build up reduces effectiveness – No items other than medicines stored in fridge (e. g. food, drink, clinical specimens) – Should not be over full – Ensure can not be accidentally switched off

Storage of Vaccines • Vaccines – must not be removed from packaging during storage – Stocks stored tidily – Not stored on shelves in fridge doors or bottom drawers – Not stored next to freezing compartments

Storage of Vaccines • Patients/Parents should not be requested to store vaccines in a domestic fridge. • Fridges should be cleaned on a regular basis • Emergency storage available if fridge fails

Temperature Monitoring • Fridges must have a reliable maximum/minimum thermometer (in addition to any integral thermometer) – Calibrate annually to ensure correct functioning • Designated person responsible for vaccine storage and fridge monitoring – Trained to read and record current temperature, maximum and minimum temperatures correctly

Temperature Monitoring • Readings should be taken daily • Keep record chart on or near the fridge • Retain records until next audit • If the recorded temperature goes outside the range, contact community services pharmacy and or the manufacturer’s for advice


Fridge Temperature Recording • NMC Standards - Registered professional has overall responsibility. • The designated person, (who shall be a professional), shall have responsibility for ensuring that the system is followed and that the security of medicines in the clinic site is maintained. • The designated person may decide to delegate some of the duties but the responsibility always remains with that designated person. • Duthie Report 2005. Community Services Pharmacy.

Vaccination Sessions • Use vaccines with shortest expiry first • Vaccines should only be removed at the beginning of session for the shortest possible time • Only remove the required number of doses for the session • Prior to administration check the identity of the vaccine, its appearance and expiry date • Record date, brand name, manufacturer, batch number and details of any diluent used in patients’ notes

Vaccination Sessions • Freeze dried vaccines should be reconstituted immediately prior to use and used within the recommended period • Any remaining vaccine in vials should be drawn into a syringe and disposed of – Part vials, prepared unused vaccines and out of date vaccines should be placed in a sharps box labelled “Vaccine waste”. The box should be sealed when two thirds full

Disposal of Vaccines • In Health Centres the box will then be either collected by pharmacy technicians or returned to community services pharmacy on secure transport • GP Practices should make arrangements for disposal of vaccine waste through their waste contractor

Spillage of Vaccines • Wear gloves and mop up with paper towels • Clean area using Sodium Hyperchlorite 1% (Milton) solution for blood and body fluid “Spill Paks” as per local policy • Dispose of contaminated waste in “Vaccine Waste” sharps box • Spillage on skin should be washed with soap and water • Affected eyes should be washed with sterile sodium chloride 0. 9% and medical advice sought • Copies of the (Control of Substances Hazardous to Health) COSHH data sheets should be obtained from the manufacturer

Disruption to the Cold Chain • Arrange for vaccines to be returned to the correct storage conditions as soon as possible • Do not use vaccines stored outside of the recommended temperature range until advice has been sought • Telephone the manufacturer for advice • Any vaccines that do not have to be discarded should be used a soon as possible

References • Department of Health: Immunisation against infectious disease 2006. The Green Book. Publication date: 30 th October 2007. • Check website www. dh. gov. uk

Neelam. ali@nottspct. nhs. uk
 Neelam ali
Neelam ali Dna vaccines pros and cons
Dna vaccines pros and cons Edible vaccines pros and cons
Edible vaccines pros and cons Advantages and disadvantages of vaccines ppt
Advantages and disadvantages of vaccines ppt Global alliance for vaccines and immunization
Global alliance for vaccines and immunization Edible vaccine definition
Edible vaccine definition Peta
Peta Neelam soundarajan
Neelam soundarajan Neelam fida
Neelam fida Neelam bhardwaja
Neelam bhardwaja Neelam poudyal
Neelam poudyal Neelam soundarajan
Neelam soundarajan Neelam soundarajan
Neelam soundarajan Glyphosate in vaccines
Glyphosate in vaccines Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Glyphosate vaccines
Glyphosate vaccines Cancer vaccines
Cancer vaccines Contamination des vaccines
Contamination des vaccines Could vaccines breed viciousness
Could vaccines breed viciousness Hep b vaccines
Hep b vaccines Live vaccines mnemonic
Live vaccines mnemonic Www.cdc.gov/vaccines/schedules/index.html
Www.cdc.gov/vaccines/schedules/index.html History of vaccines pdf
History of vaccines pdf Brighton collaboration level 1
Brighton collaboration level 1 Spacing out vaccines
Spacing out vaccines