Stoichiometry with a Twist Given the density of

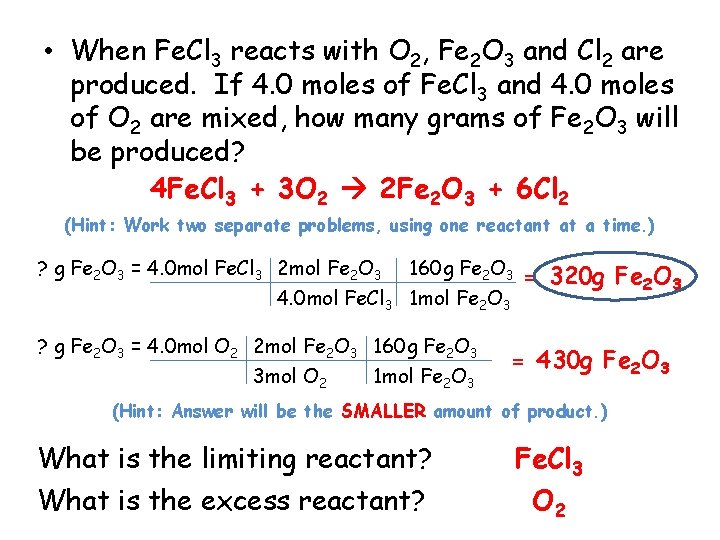
- Slides: 6

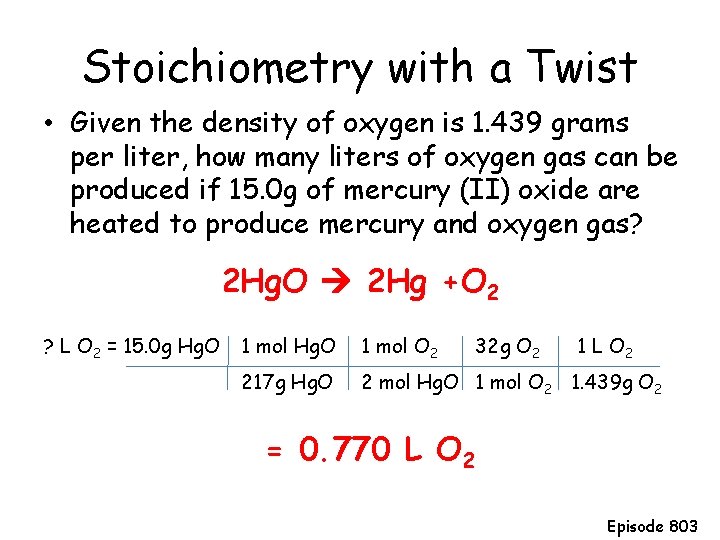
Stoichiometry with a Twist • Given the density of oxygen is 1. 439 grams per liter, how many liters of oxygen gas can be produced if 15. 0 g of mercury (II) oxide are heated to produce mercury and oxygen gas? 2 Hg. O 2 Hg +O 2 ? L O 2 = 15. 0 g Hg. O 1 mol O 2 32 g O 2 1 L O 2 217 g Hg. O 2 mol Hg. O 1 mol O 2 1. 439 g O 2 = 0. 770 L O 2 Episode 803

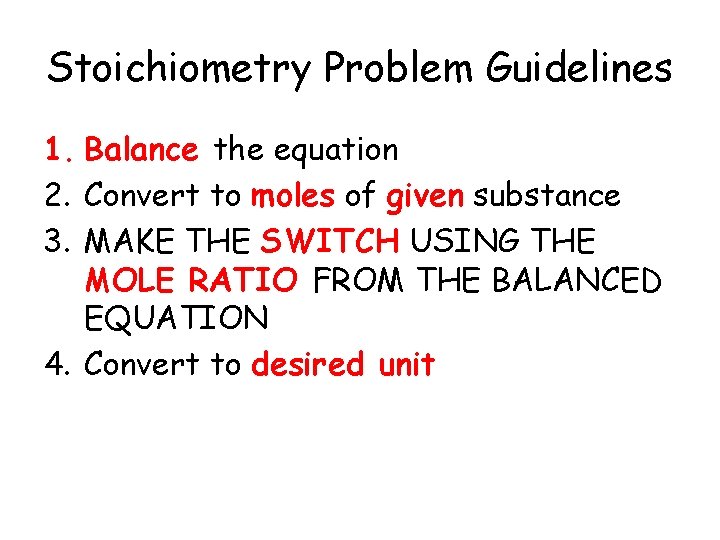
Stoichiometry Problem Guidelines 1. Balance the equation 2. Convert to moles of given substance 3. MAKE THE SWITCH USING THE MOLE RATIO FROM THE BALANCED EQUATION 4. Convert to desired unit

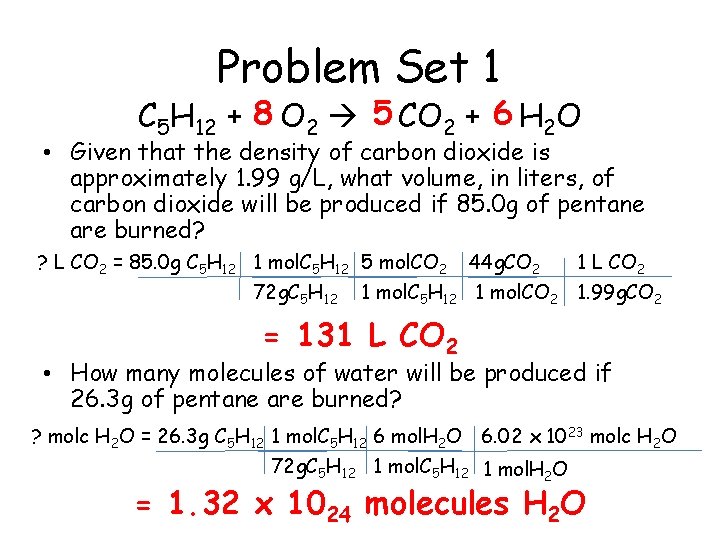
Problem Set 1 C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O • Given that the density of carbon dioxide is approximately 1. 99 g/L, what volume, in liters, of carbon dioxide will be produced if 85. 0 g of pentane are burned? ? L CO 2 = 85. 0 g C 5 H 12 1 mol. C 5 H 12 5 mol. CO 2 72 g. C 5 H 12 44 g. CO 2 1 L CO 2 1 mol. C 5 H 12 1 mol. CO 2 1. 99 g. CO 2 = 131 L CO 2 • How many molecules of water will be produced if 26. 3 g of pentane are burned? ? molc H 2 O = 26. 3 g C 5 H 12 1 mol. C 5 H 12 6 mol. H 2 O 6. 02 x 1023 molc H 2 O 72 g. C 5 H 12 1 mol. H 2 O = 1. 32 x 1024 molecules H 2 O

• Limiting Reactant – Reactant used up first in a chemical reaction – Produces the smallest amount of product • Excess Reactant – Reactant that is not used up in a chemical reaction – Leftover when the reaction is complete
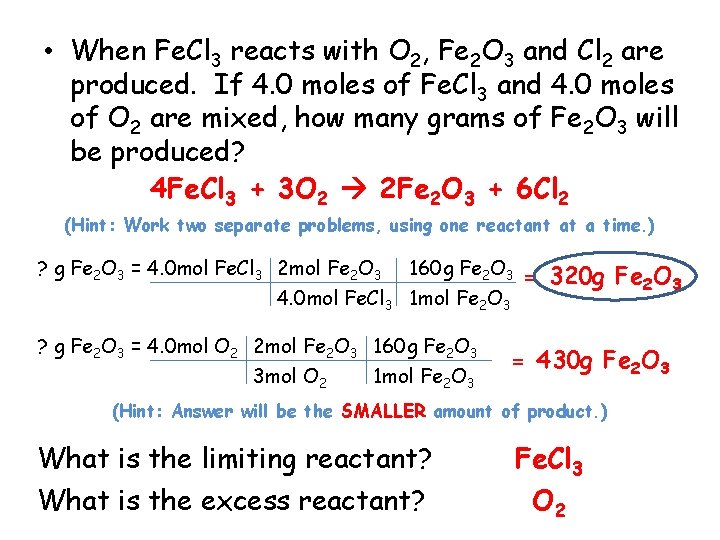
• When Fe. Cl 3 reacts with O 2, Fe 2 O 3 and Cl 2 are produced. If 4. 0 moles of Fe. Cl 3 and 4. 0 moles of O 2 are mixed, how many grams of Fe 2 O 3 will be produced? 4 Fe. Cl 3 + 3 O 2 2 Fe 2 O 3 + 6 Cl 2 (Hint: Work two separate problems, using one reactant at a time. ) ? g Fe 2 O 3 = 4. 0 mol Fe. Cl 3 2 mol Fe 2 O 3 160 g Fe 2 O 3 4. 0 mol Fe. Cl 3 1 mol Fe 2 O 3 ? g Fe 2 O 3 = 4. 0 mol O 2 2 mol Fe 2 O 3 160 g Fe 2 O 3 3 mol O 2 1 mol Fe 2 O 3 = 320 g Fe 2 O 3 = 430 g Fe 2 O 3 (Hint: Answer will be the SMALLER amount of product. ) What is the limiting reactant? What is the excess reactant? Fe. Cl 3 O 2

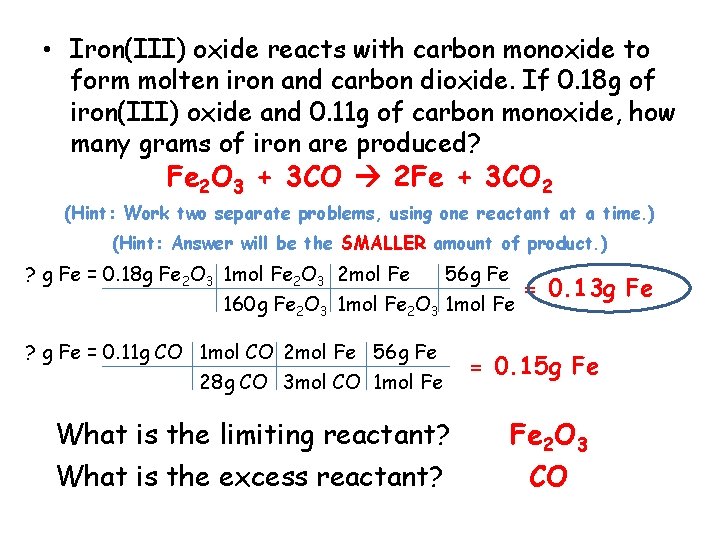
• Iron(III) oxide reacts with carbon monoxide to form molten iron and carbon dioxide. If 0. 18 g of iron(III) oxide and 0. 11 g of carbon monoxide, how many grams of iron are produced? Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 (Hint: Work two separate problems, using one reactant at a time. ) (Hint: Answer will be the SMALLER amount of product. ) ? g Fe = 0. 18 g Fe 2 O 3 1 mol Fe 2 O 3 2 mol Fe 56 g Fe 160 g Fe 2 O 3 1 mol Fe ? g Fe = 0. 11 g CO 1 mol CO 2 mol Fe 56 g Fe 28 g CO 3 mol CO 1 mol Fe What is the limiting reactant? What is the excess reactant? = 0. 13 g Fe = 0. 15 g Fe Fe 2 O 3 CO