Stoichiometry What is STOICHIOMETRY Stoichiometry refers to the














- Slides: 25

Stoichiometry

What is STOICHIOMETRY? !@!? �Stoichiometry refers to the calculations of chemical quantities from balanced chemical equations. �Allows us to relate masses of reactants and products

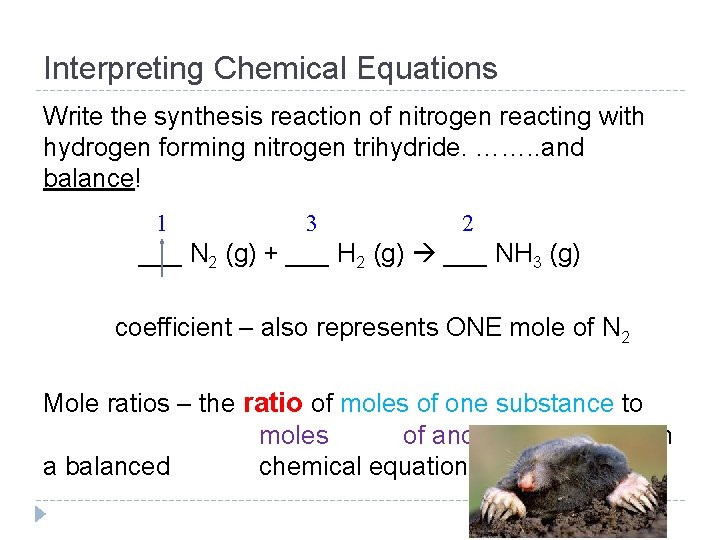
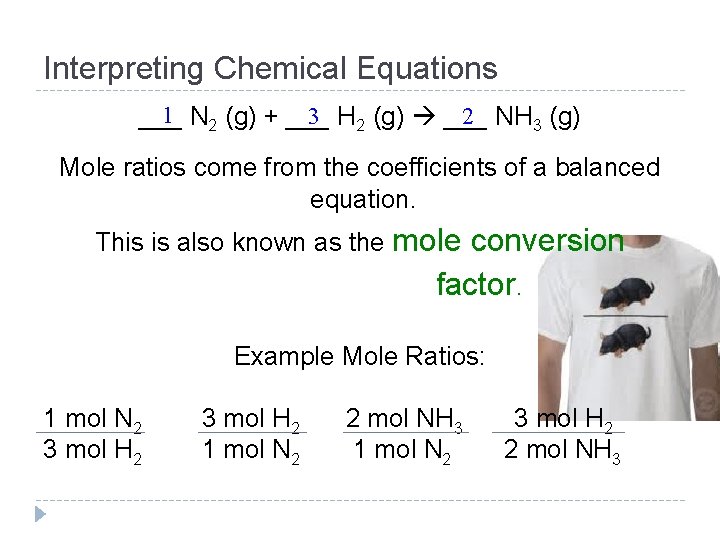
Interpreting Chemical Equations Write the synthesis reaction of nitrogen reacting with hydrogen forming nitrogen trihydride. ……. . and balance! 1 3 2 ___ N 2 (g) + ___ H 2 (g) ___ NH 3 (g) coefficient – also represents ONE mole of N 2 Mole ratios – the ratio of moles of one substance to moles of another substance in a balanced chemical equation

Interpreting Chemical Equations 1 N 2 (g) + ___ 3 H 2 (g) ___ 2 NH 3 (g) ___ Mole ratios come from the coefficients of a balanced equation. This is also known as the mole conversion factor. Example Mole Ratios: 1 mol N 2 3 mol H 2 1 mol N 2 2 mol NH 3 1 mol N 2 3 mol H 2 2 mol NH 3

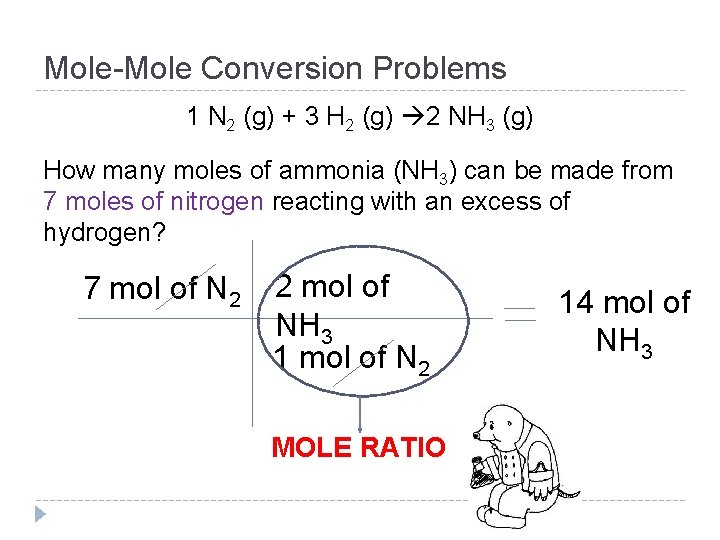
Mole-Mole Conversion Problems 1 N 2 (g) + 3 H 2 (g) 2 NH 3 (g) How many moles of ammonia (NH 3) can be made from 7 moles of nitrogen reacting with an excess of hydrogen?

Mole-Mole Conversion Problems 1 N 2 (g) + 3 H 2 (g) 2 NH 3 (g) How many moles of ammonia (NH 3) can be made from 7 moles of nitrogen reacting with an excess of hydrogen? 7 mol of N 2 2 mol of NH 3 1 mol of N 2 MOLE RATIO 14 mol of NH 3

Practice Problems How many moles of hydrogen (H 2) are required to completely react with 8 moles of nitrogen to produce ammonia (NH 3)? How many moles of nitrogen (N 2) are needed to completely react with 6 moles of hydrogen to produce ammonia (NH 3)?

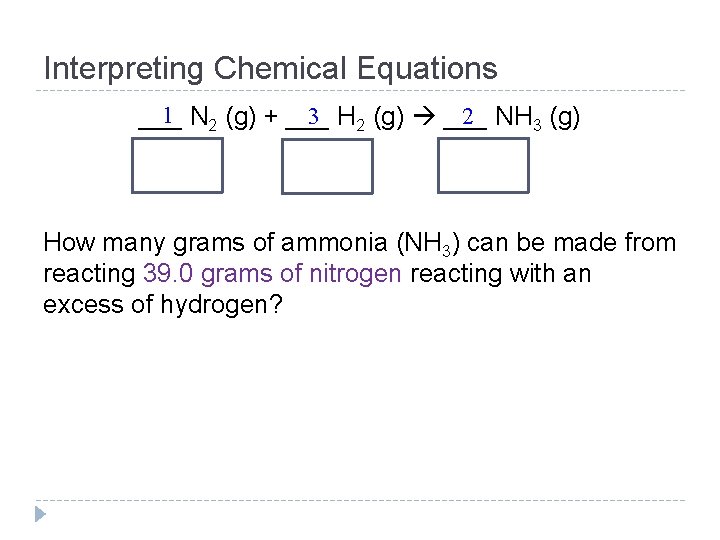
Interpreting Chemical Equations 1 N 2 (g) + ___ 3 H 2 (g) ___ 2 NH 3 (g) ___ How many grams of ammonia (NH 3) can be made from reacting 39. 0 grams of nitrogen reacting with an excess of hydrogen?

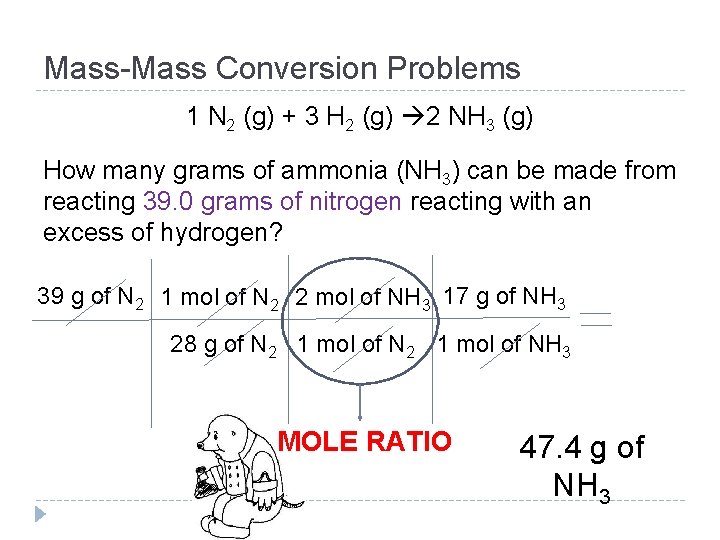
Mass-Mass Conversion Problems 1 N 2 (g) + 3 H 2 (g) 2 NH 3 (g) How many grams of ammonia (NH 3) can be made from reacting 39. 0 grams of nitrogen reacting with an excess of hydrogen? 39 g of N 2 1 mol of N 2 2 mol of NH 3 17 g of NH 3 28 g of N 2 1 mol of NH 3 MOLE RATIO 47. 4 g of NH 3

Practice Problems How many grams of H 2 are needed to react with an excess of nitrogen to make 25. 5 grams of NH 3?

Exit Slip

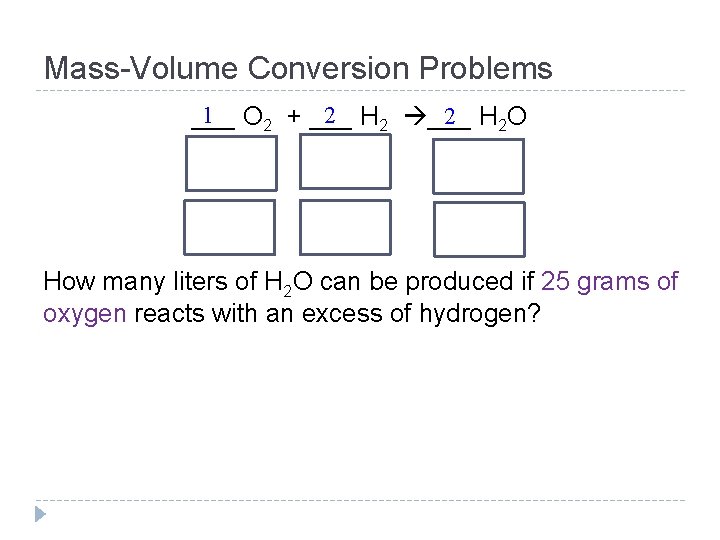
Mass-Volume Conversion Problems 1 O 2 + ___ 2 H 2 O ___ How many liters of H 2 O can be produced if 25 grams of oxygen reacts with an excess of hydrogen?

Practice Mass-Volume Problems How many grams of hydrogen are needed to completely react with an excess of oxygen to produce 35. 5 L of H 2 O?

Exit Slip

*We are assuming that Mainly because it’s easier the team is ONLY the to say “teams” over “linestarting line-up Interpreting Everyday Equations ups” 2 guards + 2 forwards + 1 center Basketball Team 2 G + 2 F + 1 C G 2 F 2 C 1) 2) 3) 14 How many guards does it take to make 7 teams? 16 How many forwards are there in 8 teams? If you have 8 centers, 17 forwards and 14 guards, how many teams can be 7 made? � What guards position do you run out of first?

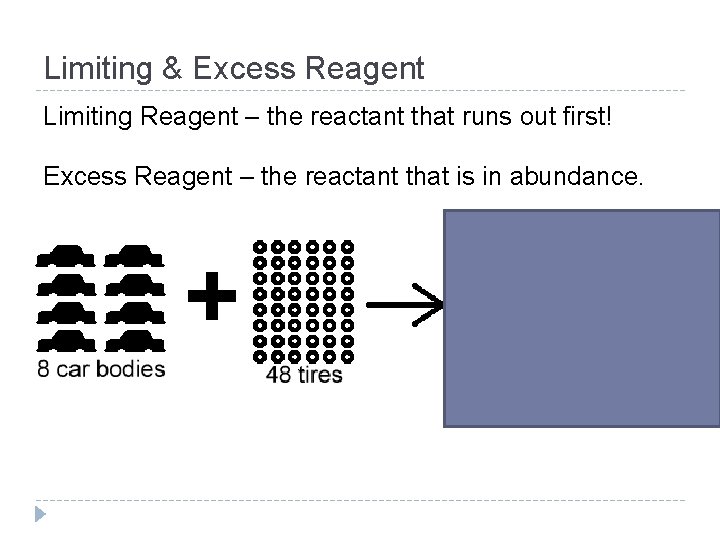
Limiting & Excess Reagent Limiting Reagent – the reactant that runs out first! Excess Reagent – the reactant that is in abundance.

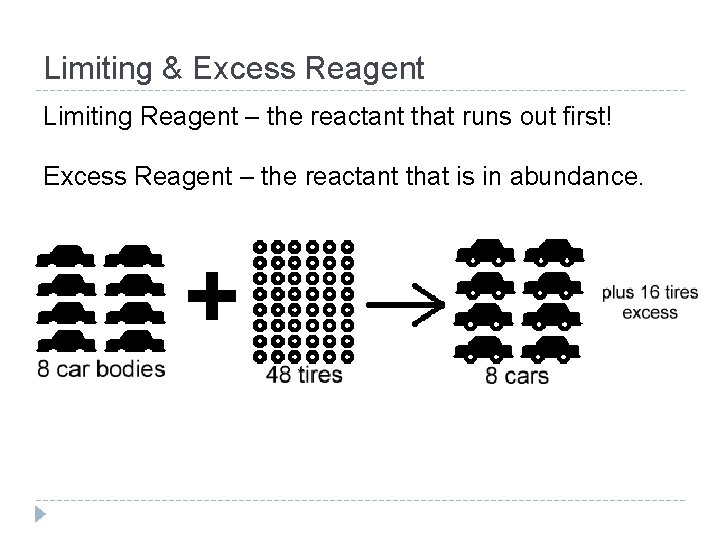
Limiting & Excess Reagent Limiting Reagent – the reactant that runs out first! Excess Reagent – the reactant that is in abundance.

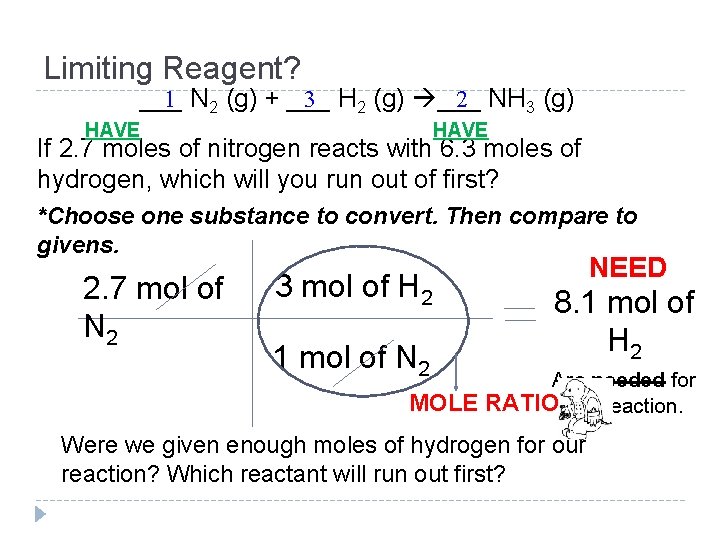
Limiting Reagent? ___ 1 N 2 (g) + ___ 3 H 2 (g) ___ 2 NH 3 (g) HAVE If 2. 7 moles of nitrogen reacts with 6. 3 moles of hydrogen, which will you run out of first? *Choose one substance to convert. Then compare to givens. 2. 7 mol of N 2 3 mol of H 2 1 mol of N 2 NEED 8. 1 mol of H 2 Are needed for MOLE RATIO this reaction. Were we given enough moles of hydrogen for our reaction? Which reactant will run out first?

You try: 1 N 2 (g) + 3 H 2 (g) 2 NH 3 (g) HAVE If 3. 9 moles of nitrogen reacts with 12. 1 moles of hydrogen, what is the limiting reagent? What would be the excess reagent? And by how much?

Exit Slip

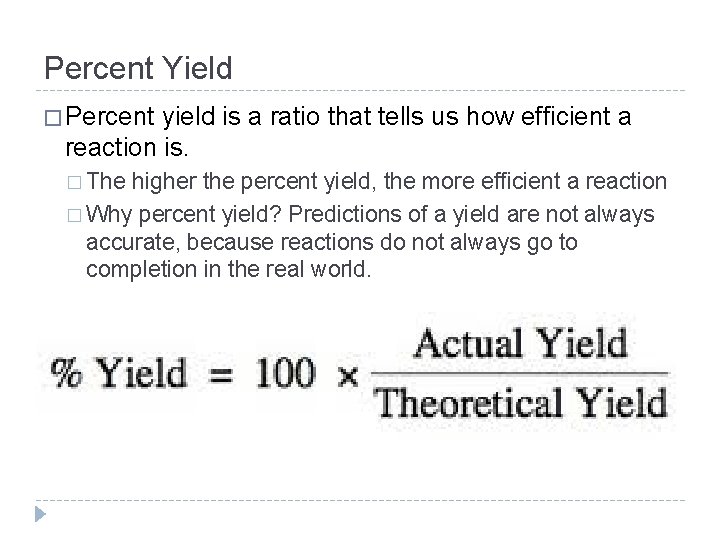
Percent Yield � Percent yield is a ratio that tells us how efficient a reaction is. � The higher the percent yield, the more efficient a reaction � Why percent yield? Predictions of a yield are not always accurate, because reactions do not always go to completion in the real world.

Percent Yield Actual Yield = the amount you experimentally get when you run a reaction in a lab. YOU ACTUALLY GOT! Theoretical Yield = the amount you are ideally supposed to get if everything goes perfectly. You can calculate this amount using. YOU stoichiometry. SHOULDA GOT!

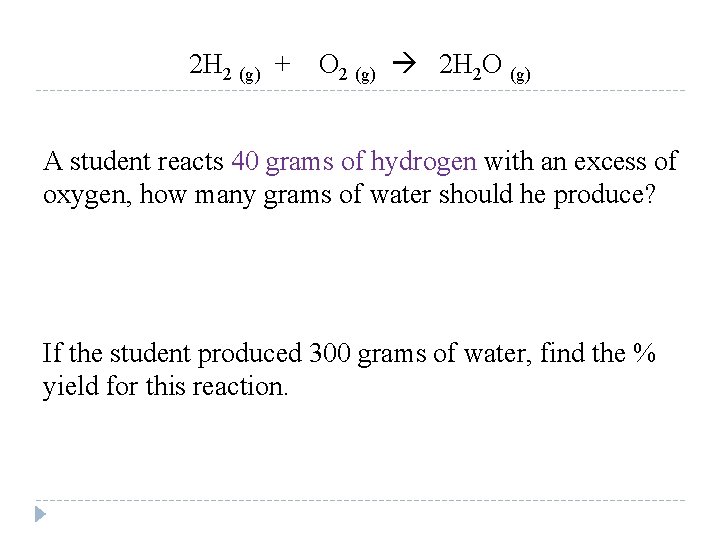
2 H 2 (g) + O 2 (g) 2 H 2 O (g) A student reacts 40 grams of hydrogen with an excess of oxygen, how many grams of water should he produce? If the student produced 300 grams of water, find the % yield for this reaction.

If 25 grams of oxygen reacted with an excess of hydrogen, how many grams of water should you produce? If you only produced 15 grams of water, what is the % yield for the reaction?

Exit Slip � When reacting oxygen and hydrogen, you produced 50 grams of water. When by using stoichiometry, you found out you should have produced 64. 5 grams of water, what is the % yield for the reaction?
 In a chemical reaction, stoichiometry refers to:
In a chemical reaction, stoichiometry refers to: Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể điện thế nghỉ
điện thế nghỉ Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ độ dài liên kết
độ dài liên kết Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Phối cảnh
Phối cảnh Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Ng-html
Ng-html Hệ hô hấp
Hệ hô hấp So nguyen to
So nguyen to đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton