STOICHIOMETRY TUTORIAL Grade 11 Chemistry Mr Krstovic 1

- Slides: 13

STOICHIOMETRY TUTORIAL Grade 11 Chemistry Mr. Krstovic 1

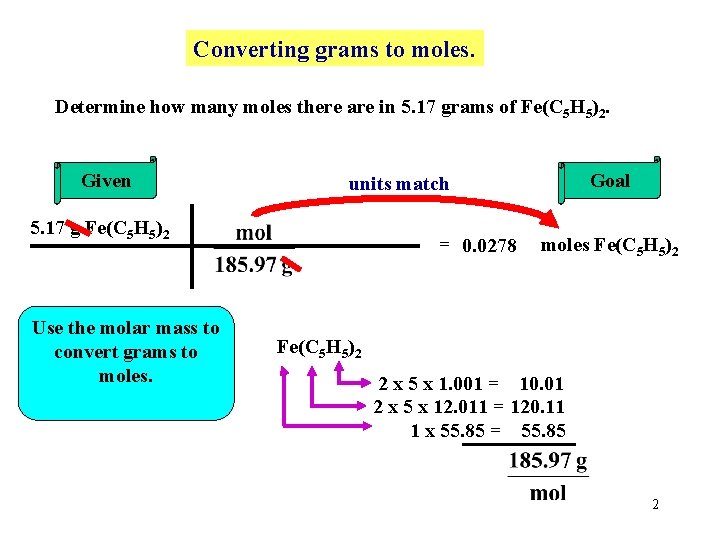
Converting grams to moles. Determine how many moles there are in 5. 17 grams of Fe(C 5 H 5)2. Given 5. 17 g Fe(C 5 H 5)2 Use the molar mass to convert grams to moles. Goal units match = 0. 0278 moles Fe(C 5 H 5)2 2 x 5 x 1. 001 = 10. 01 2 x 5 x 12. 011 = 120. 11 1 x 55. 85 = 55. 85 2

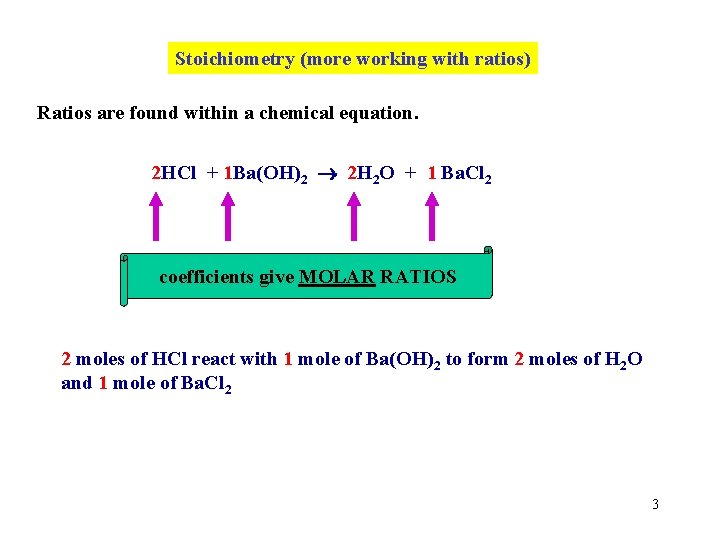
Stoichiometry (more working with ratios) Ratios are found within a chemical equation. 2 HCl + 1 Ba(OH)2 2 H 2 O + 1 Ba. Cl 2 coefficients give MOLAR RATIOS 2 moles of HCl react with 1 mole of Ba(OH)2 to form 2 moles of H 2 O and 1 mole of Ba. Cl 2 3

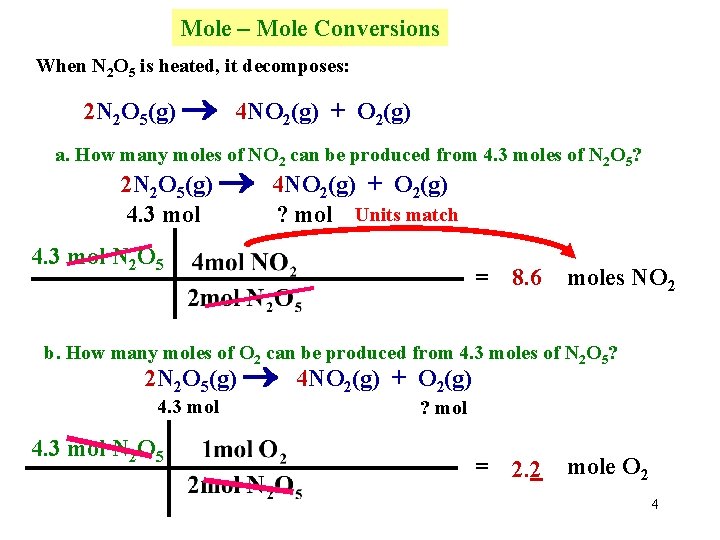
Mole – Mole Conversions When N 2 O 5 is heated, it decomposes: 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) a. How many moles of NO 2 can be produced from 4. 3 moles of N 2 O 5? 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) 4. 3 mol ? mol Units match 4. 3 mol N 2 O 5 = 8. 6 moles NO 2 b. How many moles of O 2 can be produced from 4. 3 moles of N 2 O 5? 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) 4. 3 mol N 2 O 5 ? mol = 2. 2 mole O 2 4

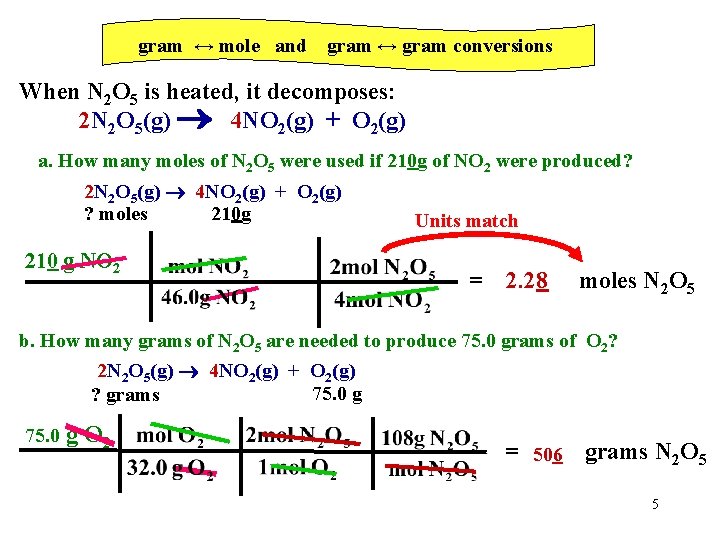
gram ↔ mole and gram ↔ gram conversions When N 2 O 5 is heated, it decomposes: 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) a. How many moles of N 2 O 5 were used if 210 g of NO 2 were produced? 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) ? moles 210 g 210 g NO 2 Units match = 2. 28 moles N 2 O 5 b. How many grams of N 2 O 5 are needed to produce 75. 0 grams of O 2? 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) 75. 0 g ? grams 75. 0 g O 2 = 506 grams N 2 O 5 5

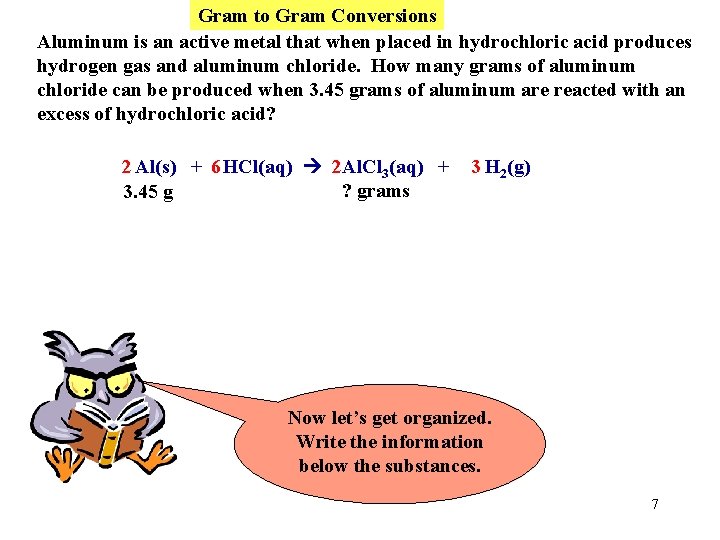
Gram to Gram Conversions Aluminum is an active metal that when placed in hydrochloric acid produces hydrogen gas and aluminum chloride. How many grams of aluminum chloride can be produced when 3. 45 grams of aluminum are reacted with an excess of hydrochloric acid? 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + 3 H 2(g) First write a balanced equation. 6

Gram to Gram Conversions Aluminum is an active metal that when placed in hydrochloric acid produces hydrogen gas and aluminum chloride. How many grams of aluminum chloride can be produced when 3. 45 grams of aluminum are reacted with an excess of hydrochloric acid? 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + ? grams 3. 45 g 3 H 2(g) Now let’s get organized. Write the information below the substances. 7

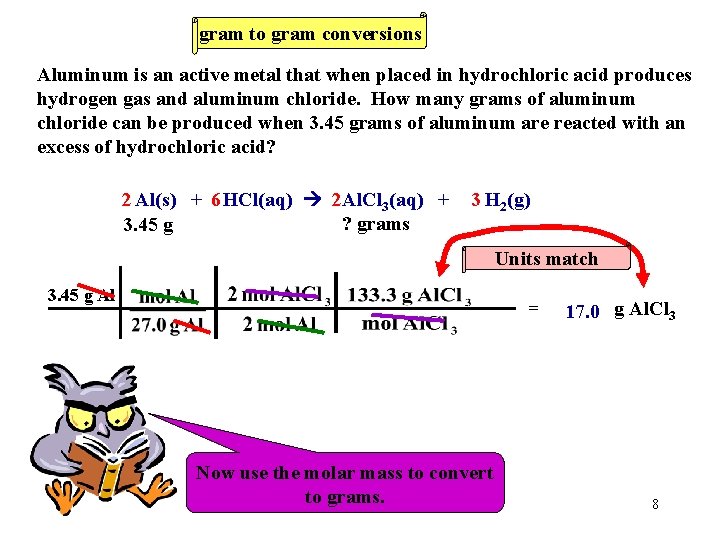
gram to gram conversions Aluminum is an active metal that when placed in hydrochloric acid produces hydrogen gas and aluminum chloride. How many grams of aluminum chloride can be produced when 3. 45 grams of aluminum are reacted with an excess of hydrochloric acid? 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + ? grams 3. 45 g 3 H 2(g) Units match 3. 45 g Al = Now We must Now use Let’s the always use work molar thethe convert molar mass problem. ratio. to toconvert moles. to grams. 17. 0 g Al. Cl 3 8

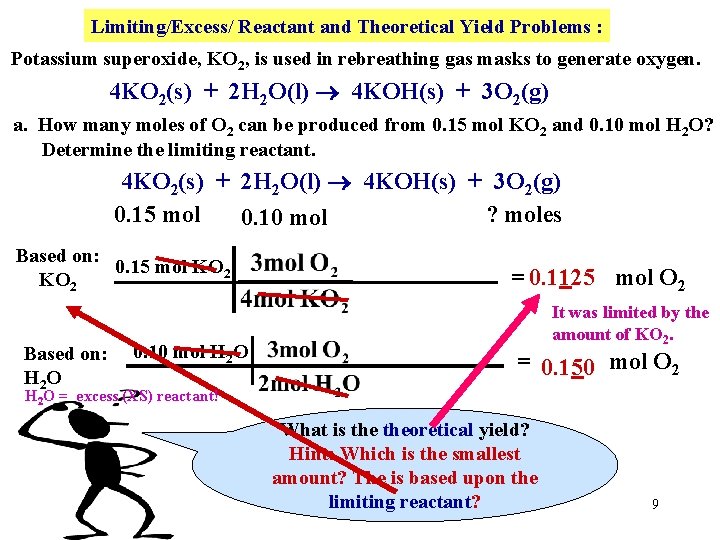
Limiting/Excess/ Reactant and Theoretical Yield Problems : Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) 0. 15 mol ? moles 0. 10 mol Based on: 0. 15 mol KO 2 Based on: H 2 O 0. 10 mol H 2 O = 0. 1125 mol O 2 It was limited by the amount of KO 2. = 0. 150 mol O 2 H 2 O = excess (XS) reactant! What is theoretical yield? Hint: Which is the smallest amount? The is based upon the limiting reactant? 9

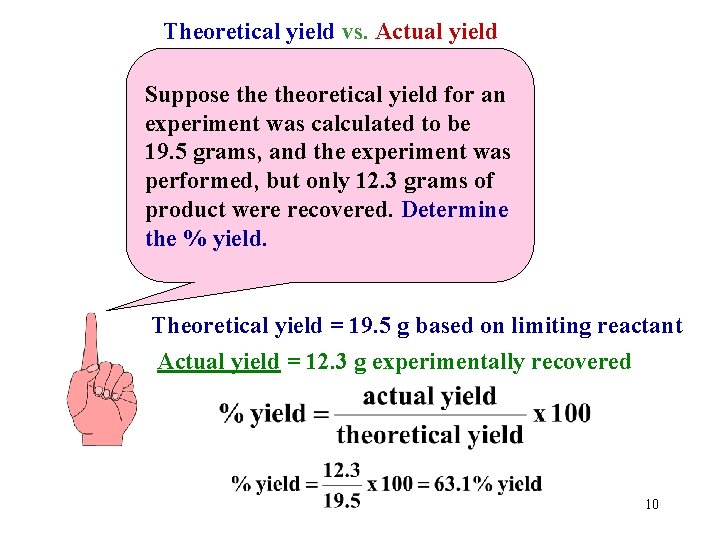
Theoretical yield vs. Actual yield Suppose theoretical yield for an experiment was calculated to be 19. 5 grams, and the experiment was performed, but only 12. 3 grams of product were recovered. Determine the % yield. Theoretical yield = 19. 5 g based on limiting reactant Actual yield = 12. 3 g experimentally recovered 10

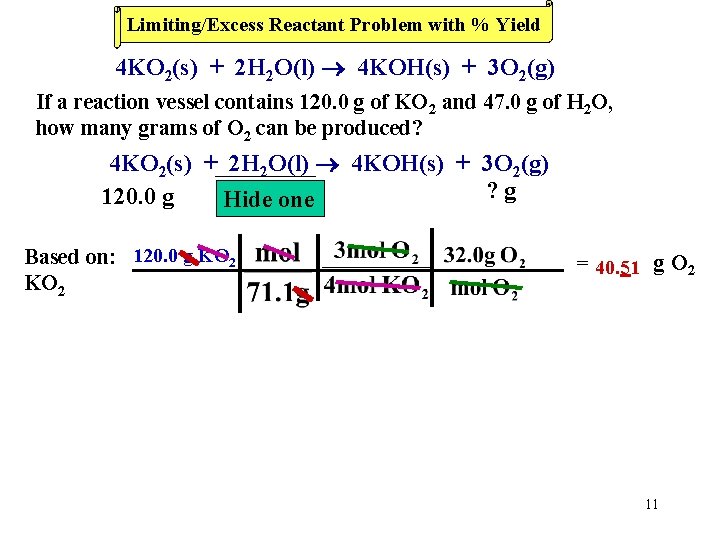
Limiting/Excess Reactant Problem with % Yield 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 g 47. 0 one g Hide Based on: 120. 0 g KO 2 = 40. 51 g O 2 11

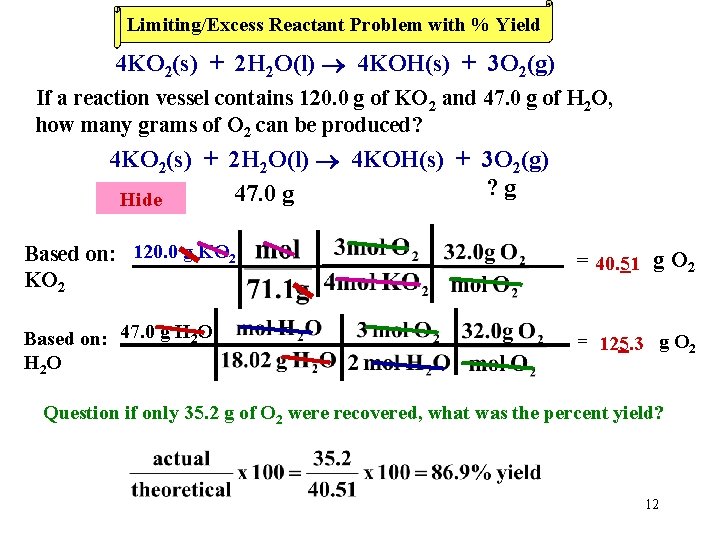
Limiting/Excess Reactant Problem with % Yield 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 47. 0 g Hideg Based on: 120. 0 g KO 2 = 40. 51 g O 2 Based on: 47. 0 g H 2 O H 2 O = 125. 3 g O 2 Question if only 35. 2 g of O 2 were recovered, what was the percent yield? 12

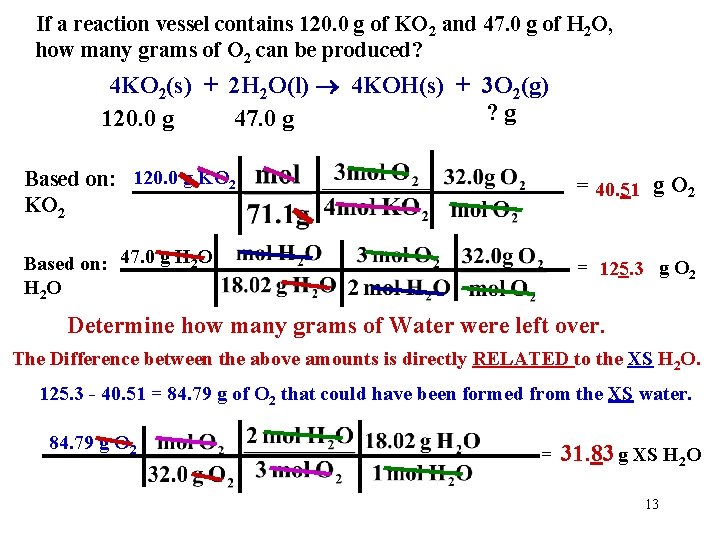
If a reaction vessel contains 120. 0 g of KO 2 and 47. 0 g of H 2 O, how many grams of O 2 can be produced? 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) ? g 120. 0 g 47. 0 g Based on: 120. 0 g KO 2 = 40. 51 g O 2 Based on: 47. 0 g H 2 O H 2 O = 125. 3 g O 2 Determine how many grams of Water were left over. The Difference between the above amounts is directly RELATED to the XS H 2 O. 125. 3 - 40. 51 = 84. 79 g of O 2 that could have been formed from the XS water. 84. 79 g O 2 = 31. 83 g XS H 2 O 13