Stoichiometry The Mole What is a mole No







- Slides: 20

Stoichiometry

The Mole �What is a mole?

No not this type of mole either

The Mole �Chemists use a measurement called ‘the mole’ �A mole is like a dozen eggs= 12 eggs or a pair of shoes= 2 shoes �However, the mole is a very large number This is because atoms are so small that chemists need a lot to be able to find their mass

How big is the mole? ? �A mole contains 6. 023 x 1023 of anything 6. 023 x 1023 is a. k. a AVOGADRO’S NUMBER �How big is a mole exactly? If you could count 10 million numbers every second, it would take you 2 billion years to count an avogadro’s number out If you had a mole of unpopped popcorn kernals it would cover the entire planet to a depth of 9 miles If you poured out a mole number of cans of pop out you would cover the entire earth with an ocean of pop 200 miles deep

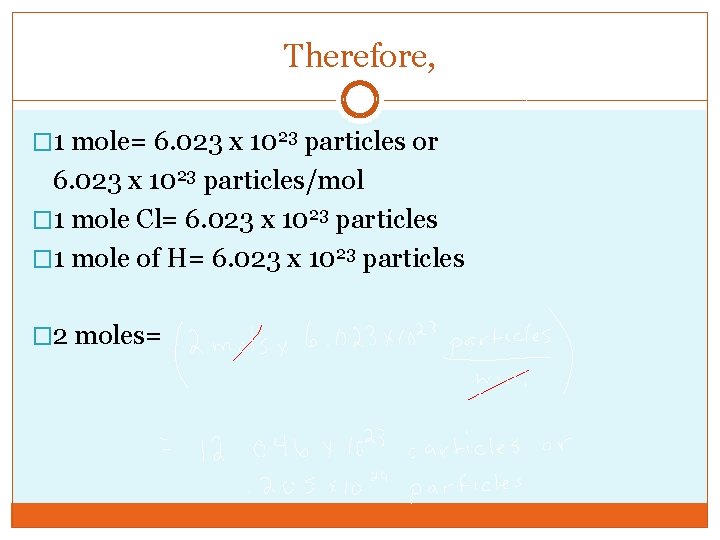
Therefore, � 1 mole= 6. 023 x 1023 particles or 6. 023 x 1023 particles/mol � 1 mole Cl= 6. 023 x 1023 particles � 1 mole of H= 6. 023 x 1023 particles � 2 moles=

Atomic Molar Mass �The mass (in grams) of 1 mole of a given element �To find this for each element you must look at the periodic table: 1 mole of C= 12 g (or 12 g/mol) What is the mass of 1 mole of oxygen? What about 2 moles of oxygen? 3 moles?

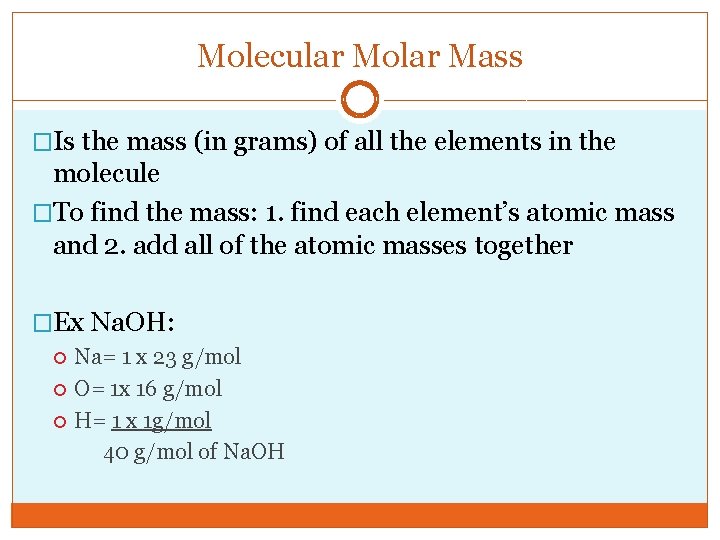
Molecular Molar Mass �Is the mass (in grams) of all the elements in the molecule �To find the mass: 1. find each element’s atomic mass and 2. add all of the atomic masses together �Ex Na. OH: Na= 1 x 23 g/mol O= 1 x 16 g/mol H= 1 x 1 g/mol 40 g/mol of Na. OH

�Ex H 2 O �Ex. H 2 SO 4

Find the following masses �Mg. Br 2, Ga(NO 2)3, Cr. I 2, Be. SO 4 �Assignment: molar mass worksheet

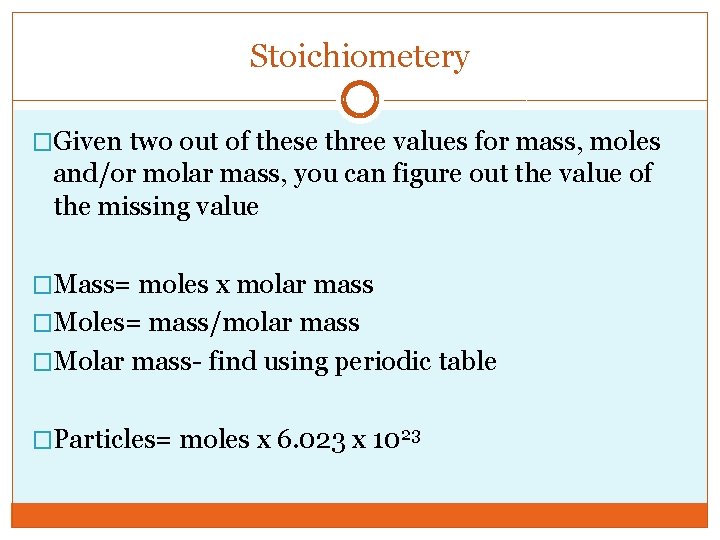
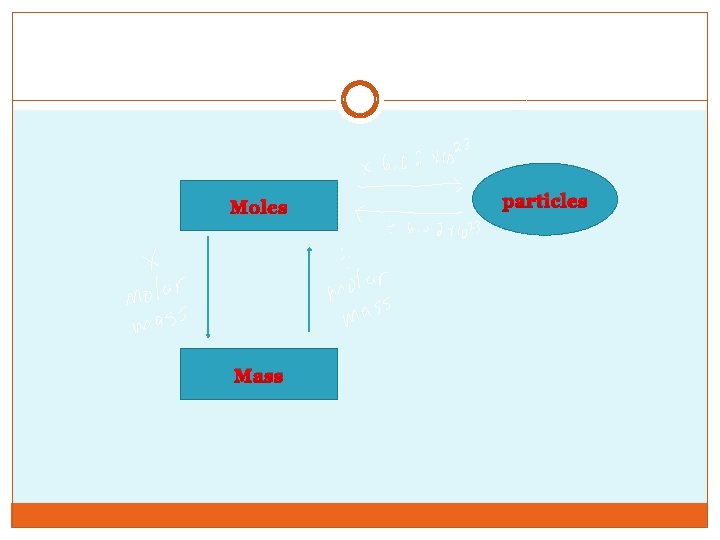
Stoichiometery �Given two out of these three values for mass, moles and/or molar mass, you can figure out the value of the missing value �Mass= moles x molar mass �Moles= mass/molar mass �Molar mass- find using periodic table �Particles= moles x 6. 023 x 1023

Moles Mass particles

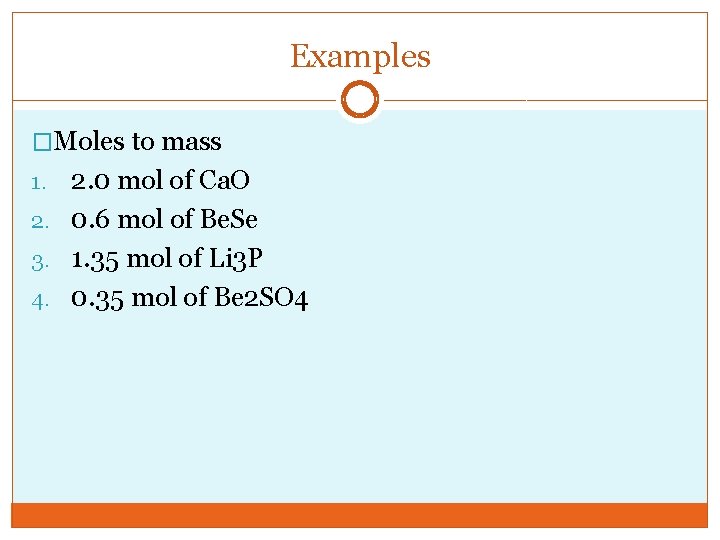
Examples �Moles to mass 2. 0 mol of Ca. O 2. 0. 6 mol of Be. Se 3. 1. 35 mol of Li 3 P 4. 0. 35 mol of Be 2 SO 4 1.

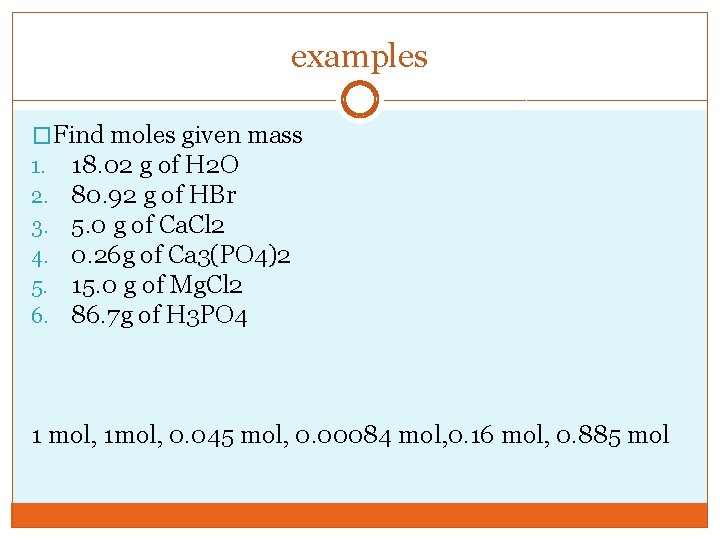
examples �Find moles given mass 1. 18. 02 g of H 2 O 2. 80. 92 g of HBr 3. 5. 0 g of Ca. Cl 2 4. 0. 26 g of Ca 3(PO 4)2 5. 15. 0 g of Mg. Cl 2 6. 86. 7 g of H 3 PO 4 1 mol, 1 mol, 0. 045 mol, 0. 00084 mol, 0. 16 mol, 0. 885 mol

Assignments �Assignment: molar mass/ moles/mass assignment

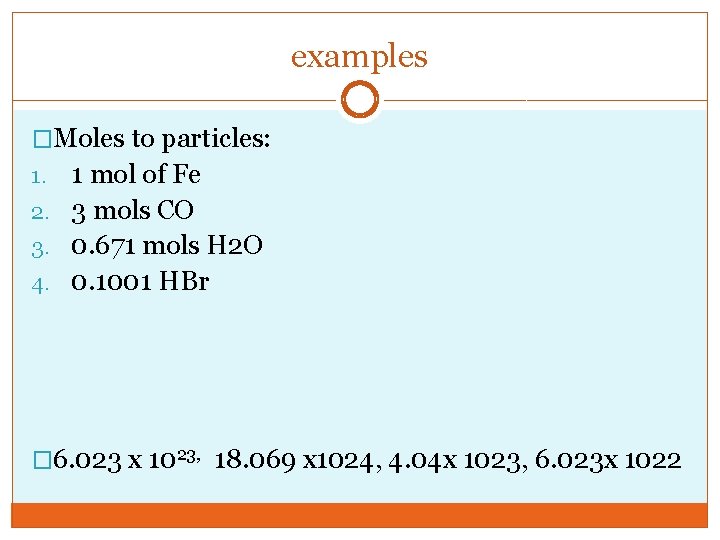
examples �Moles to particles: 1 mol of Fe 2. 3 mols CO 3. 0. 671 mols H 2 O 4. 0. 1001 HBr 1. � 6. 023 x 1023, 18. 069 x 1024, 4. 04 x 1023, 6. 023 x 1022

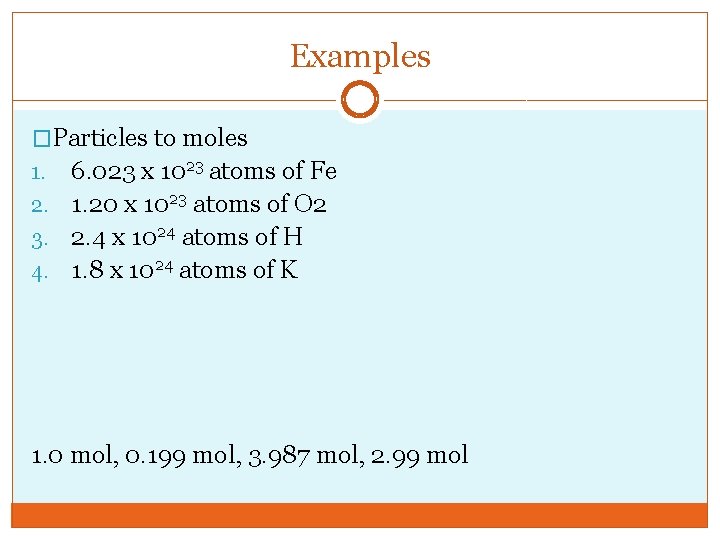
Examples �Particles to moles 6. 023 x 1023 atoms of Fe 2. 1. 20 x 1023 atoms of O 2 3. 2. 4 x 1024 atoms of H 4. 1. 8 x 1024 atoms of K 1. 0 mol, 0. 199 mol, 3. 987 mol, 2. 99 mol

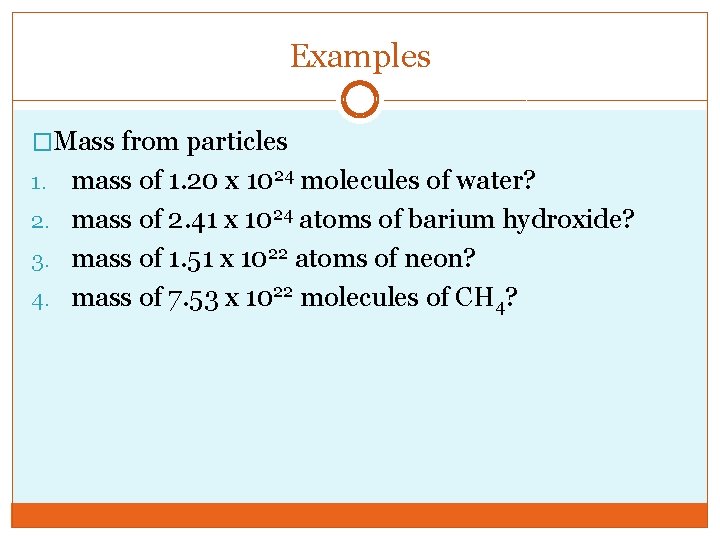
Examples �Mass from particles mass of 1. 20 x 1024 molecules of water? 2. mass of 2. 41 x 1024 atoms of barium hydroxide? 3. mass of 1. 51 x 1022 atoms of neon? 4. mass of 7. 53 x 1022 molecules of CH 4? 1.

�Particles from mass 1. How many molecules does 36. 0 grams of water represent? 2. How many molecules does 11. 0 grams of CO 2 represent? 3. How many atoms does 3. 0 grams of carbon represent? 4. How many formula units does 200. 0 grams of calcium carbonate represent?

assignment �Stoichiometery problems with # of molecules � Stoichiometery assignment with all the examples