Stoichiometry The MOLE RATIO In a balanced chemical

Stoichiometry

The MOLE RATIO �In a balanced chemical reaction, the coefficients tell you how many moles of each substance you need for the reaction and how many moles of each product you will produce (theoretically). �C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O � 1 mol C 3 H 8 + 5 mol O 2 3 mol CO 2 + 4 mol H 2 O

What is the MOLE RATIO used for? �The mole ratio is used to convert from one substance to another. (Dimensional Analysis style of course!) We use this because we don’t have any instruments that measure things in MOLES!
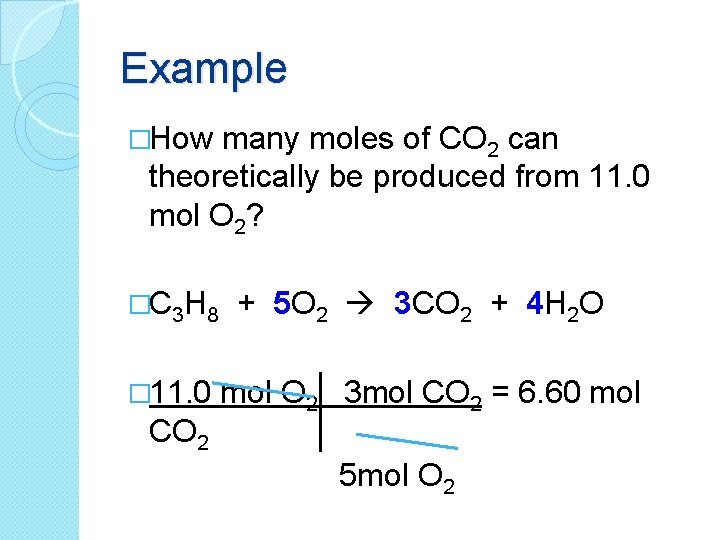
Example �How many moles of CO 2 can theoretically be produced from 11. 0 mol O 2? �C 3 H 8 � 11. 0 CO 2 + 5 O 2 3 CO 2 + 4 H 2 O mol O 2 3 mol CO 2 = 6. 60 mol 5 mol O 2

PAUSE NOW TO TRY SOME EXAMPLES WITH YOUR FAVORITE CHEMISTRY TEACHER!

�Of course, we don’t measure things in moles and most of the time our answers aren’t in moles either. �We measure substances in… ◦ Mass (grams) ◦ Volume (m. L) ◦ Molecules/Atoms �Lucky for us, we know how to convert all of those into and out of moles!

2 HCl + Ca. CO 3 CO 2 + H 2 O + 2 Ca. Cl 2 �How many moles of calcium chloride are produced from 100. 0 g of blackboard chalk, calcium carbonate? ◦ What are we given in the problem? �Balanced equation � 100. 0 g of Ca. CO 3 ◦ What are we trying to find? �Moles of Ca. Cl 2 ◦ What should we do 1 st?
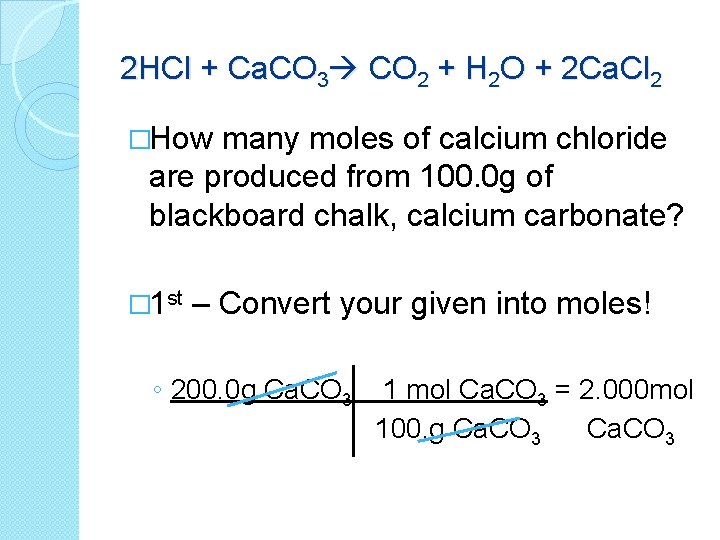
2 HCl + Ca. CO 3 CO 2 + H 2 O + 2 Ca. Cl 2 �How many moles of calcium chloride are produced from 100. 0 g of blackboard chalk, calcium carbonate? � 1 st – Convert your given into moles! ◦ 200. 0 g Ca. CO 3 1 mol Ca. CO 3 = 2. 000 mol 100. g Ca. CO 3

2 HCl + Ca. CO 3 CO 2 + H 2 O + 2 Ca. Cl 2 �How many moles of calcium chloride are produced from 200. 0 g of blackboard chalk, calcium carbonate? �Then, take that answer and use it with your mole ratio! � 2. 000 mol Ca. CO 3 2 mol Ca. Cl 2 = 4. 000 mol 1 mol Ca. CO 3 Ca. Cl 2
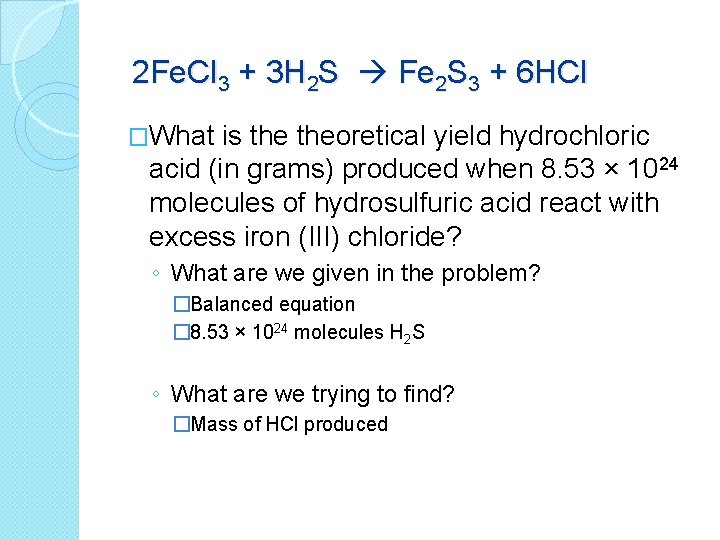
2 Fe. Cl 3 + 3 H 2 S Fe 2 S 3 + 6 HCl �What is theoretical yield hydrochloric acid (in grams) produced when 8. 53 × 1024 molecules of hydrosulfuric acid react with excess iron (III) chloride? ◦ What are we given in the problem? �Balanced equation � 8. 53 × 1024 molecules H 2 S ◦ What are we trying to find? �Mass of HCl produced
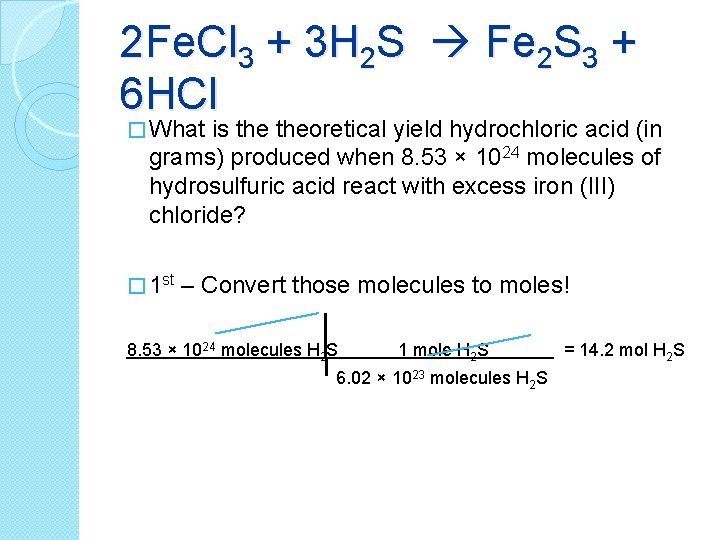
2 Fe. Cl 3 + 3 H 2 S Fe 2 S 3 + 6 HCl � What is theoretical yield hydrochloric acid (in grams) produced when 8. 53 × 1024 molecules of hydrosulfuric acid react with excess iron (III) chloride? � 1 st – Convert those molecules to moles! 8. 53 × 1024 molecules H 2 S 1 mole H 2 S 6. 02 × 1023 molecules H 2 S = 14. 2 mol H 2 S
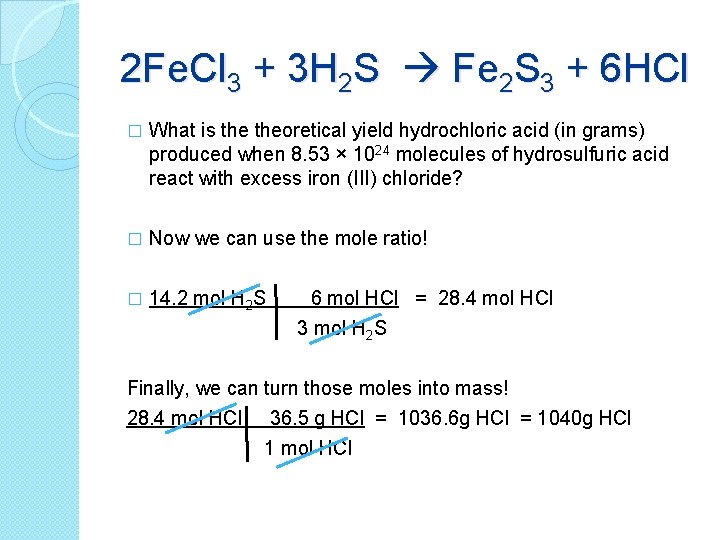
2 Fe. Cl 3 + 3 H 2 S Fe 2 S 3 + 6 HCl � What is theoretical yield hydrochloric acid (in grams) produced when 8. 53 × 1024 molecules of hydrosulfuric acid react with excess iron (III) chloride? � Now we can use the mole ratio! � 14. 2 mol H 2 S 6 mol HCl = 28. 4 mol HCl 3 mol H 2 S Finally, we can turn those moles into mass! 28. 4 mol HCl 36. 5 g HCl = 1036. 6 g HCl = 1040 g HCl 1 mol HCl
- Slides: 12