Stoichiometry Stoichiometry The process of using a balanced

Stoichiometry

Stoichiometry • The process of using a balanced chemical equation to determine the relative amounts of reactants and products involved in a reaction.

What do Chemical Equations Tell Us? • Chemical changes involve the rearrangements of atom groupings as one or more substances change to new substances (like rearranging your room) • Reactants vs. Products

What does this reaction tell us? CO(g) + 2 H 2(g) CH 3 OH(l) The coefficients can be used to describe the reaction The coefficients can have units of moles or molecules 1 molecule CO + 2 molecules H 2 1 molecule CH 3 OH 6. 02 x 1023 CO molecules + 2 (6. 02 x 1023) H 2 molecules 6. 02 x 1023 CH 3 OH molecules 1 mol CO molecules + 2 mol H 2 molecules 1 mol CH 3 OH molecules
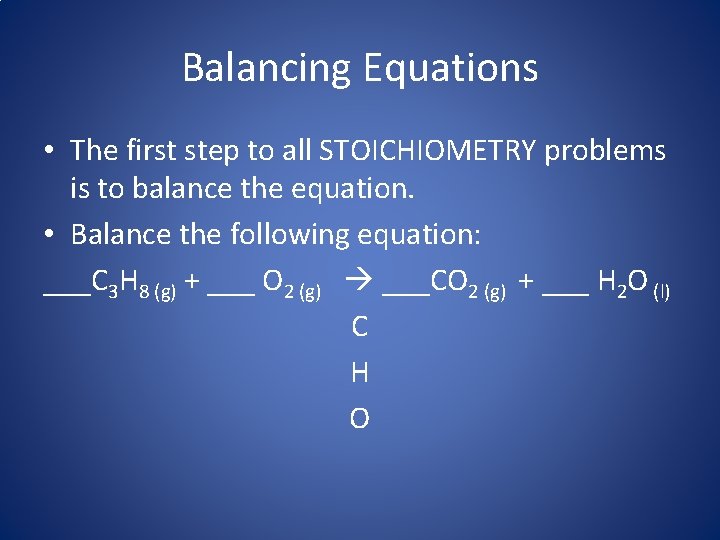
Balancing Equations • The first step to all STOICHIOMETRY problems is to balance the equation. • Balance the following equation: ___C 3 H 8 (g) + ___ O 2 (g) ___CO 2 (g) + ___ H 2 O (l) C H O
Mole Ratio • Mole ratio= the ratio of moles of one substance to moles of another substance in a balanced chemical equation • Ex: 2 Na + Cl 2 2 Na. Cl • What is the mole ratio of sodium to chlorine gas? • What is the mole ratio of sodium to sodium chloride? • What is the mole ratio of chlorine to sodium chloride? • Why is a BALANCED chemical equation important? • In stoichiometry mole ratios are used as conversion factors.
Mole to Mole Relationships • We can used a balanced chemical equation to predict the moles of products that a given number of moles of reactant will yield, or moles of reactant needed for a certain amount of product • Moles are used instead of mass because each element/compound has a different mass • A balanced equation is needed to make a mole to mole comparison. • The coefficients in a balanced equation will give us the mole to mole ratio • Mole ratios are used as conversion factors

Mole to Mole Example #1 •

Mole to Mole Example #2 •

Mole to Mole Ex. 3 • For a synthesis reaction between hydrogen and oxygen to make water answer the following: • A) How many moles of H 2 O are produced when 5. 00 moles of oxygen are used? • B) If 3. 00 moles of H 2 O are produced, how many moles of oxygen are needed? • C) How many moles of hydrogen gas are needed to produce 4 moles of water? • A) 10. 0 moles of H 2 O • B) 1. 50 moles of O 2 • C) 4 moles of H 2
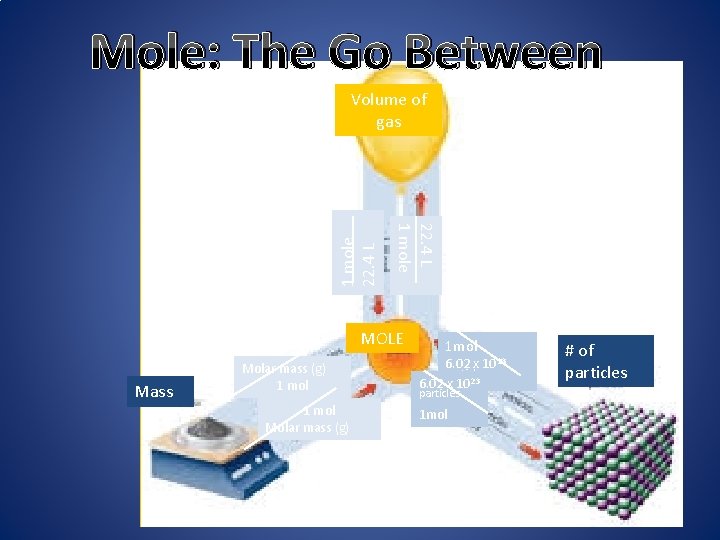
Mole: The Go Between 22. 4 L 1 mole 22. 4 L Volume of gas MOLE Mass Molar mass (g) 1 mol Molar mass (g) 1 mol 6. 02 x 1023 particles 1 mol # of particles
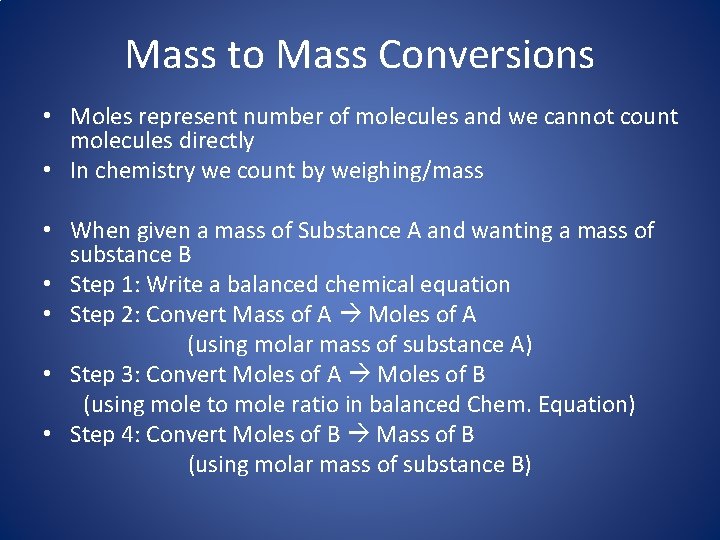
Mass to Mass Conversions • Moles represent number of molecules and we cannot count molecules directly • In chemistry we count by weighing/mass • When given a mass of Substance A and wanting a mass of substance B • Step 1: Write a balanced chemical equation • Step 2: Convert Mass of A Moles of A (using molar mass of substance A) • Step 3: Convert Moles of A Moles of B (using mole to mole ratio in balanced Chem. Equation) • Step 4: Convert Moles of B Mass of B (using molar mass of substance B)
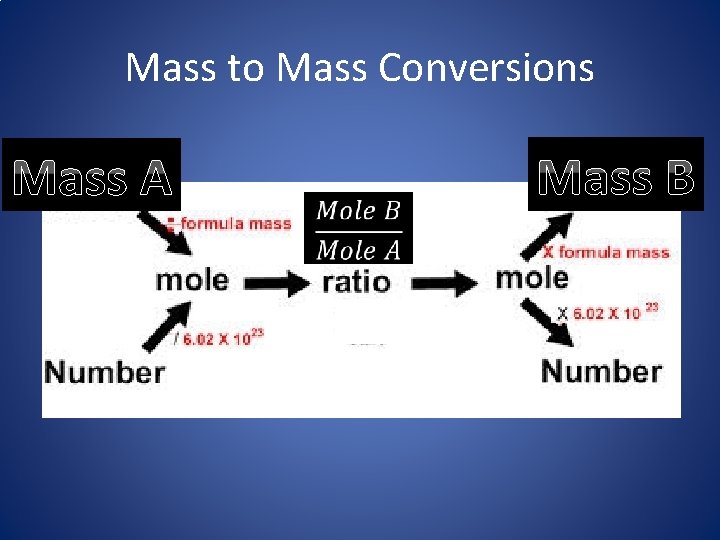
Mass to Mass Conversions Mass A Mass B

Mass to Mass Conversion Ex. 4 • Problem: Oxygen gas can be produced by decomposing potassium chlorate (potassium chloride is also produced). If 138. 6 g of KCl. O 3 is heated and decomposes completely, what mass of oxygen gas is produced? • How do we solve? – Always start with a balanced chemical equation – We are given the # grams of potassium chlorate, so we must convert to moles of potassium chlorate because the balanced equation deals in moles rather than grams – Next we can use the coefficients in the balanced equation to determine the mole ratio – Finally, we will use the molar mass of oxygen gas to calculate grams of oxygen gas produced
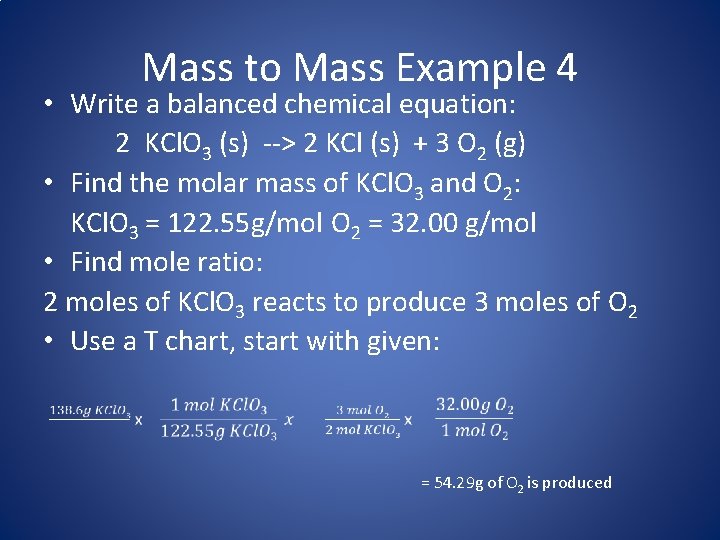
Mass to Mass Example 4 • Write a balanced chemical equation: 2 KCl. O 3 (s) --> 2 KCl (s) + 3 O 2 (g) • Find the molar mass of KCl. O 3 and O 2: KCl. O 3 = 122. 55 g/mol O 2 = 32. 00 g/mol • Find mole ratio: 2 moles of KCl. O 3 reacts to produce 3 moles of O 2 • Use a T chart, start with given: = 54. 29 g of O 2 is produced

Mass to Mass Ex. 5 • A chemical reaction between diboron hexahydride and oxygen gas will produce Oxoborinic acid (HBO 2)and water. A. ) What mass of O 2 will be needed to burn 36. 1 g of diboron hexahydride? B. ) How many moles of water are produced from 19. 2 g of B 2 H 6? • What is always the first step? • What is given? • What do we want to determine? (there are two questions in one) • What information do we need to know? • What is the mole ratio?

Answer: Practice Problem •

Molecules

S’mores • What is the “formula” for making s’mores? How many s’mores could I make if I had 8 gram crackers? How many s’mores could I make if I had 8 marshmallows? How many s’mores could I make if I had 8 chocolate squares? If I have 8 gram crackers, 8 marshmallows, and 8 chocolate squares, how many s’mores could I make? • Which ingredient limits the amount of s’mores that can be made? • Which ingredients are excess (left over? )? How much excess of those ingredients would you have? • •
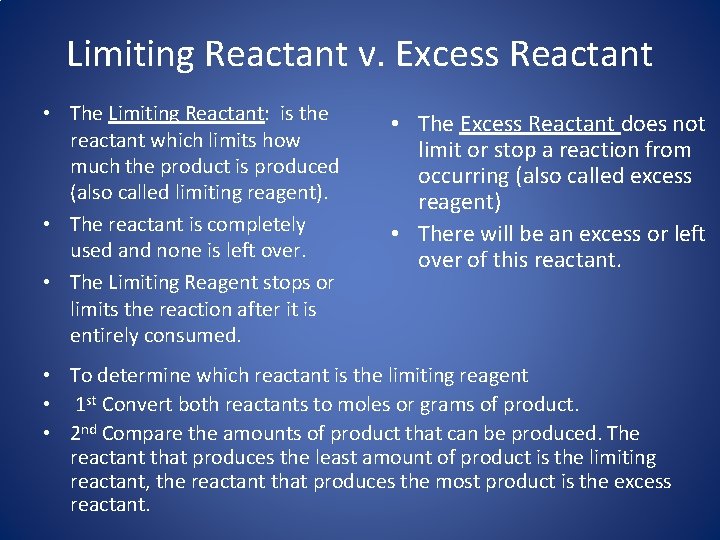
Limiting Reactant v. Excess Reactant • The Limiting Reactant: is the reactant which limits how much the product is produced (also called limiting reagent). • The reactant is completely used and none is left over. • The Limiting Reagent stops or limits the reaction after it is entirely consumed. • The Excess Reactant does not limit or stop a reaction from occurring (also called excess reagent) • There will be an excess or left over of this reactant. • To determine which reactant is the limiting reagent • 1 st Convert both reactants to moles or grams of product. • 2 nd Compare the amounts of product that can be produced. The reactant that produces the least amount of product is the limiting reactant, the reactant that produces the most product is the excess reactant.

Limiting Reagent Example # 9 Problem: Aluminum reacts with chlorine to form aluminum chloride 2 Al + 3 Cl 2 ---> 2 Al. Cl 3 If you have 34. 0 g of Al and 39. 0 g of Cl 2, what is the limiting reactant? What is the excess reactant? •

Limiting Reagent Example #10 Problem: When 3. 22 moles of Al reacts with 4. 96 moles of HBr, how many moles of H 2 are formed, considering the reaction below? What is the limiting reactant and the excess reactatnt? 2 Al + 6 HBr → 2 Al. Br 3 + 3 H 2 Part 1: • Al: 3. 22 mol Al 3 mol H 2 = 2 mol Al • HBr: 4. 96 mol HBr 3 mol H 2 = 4. 83 mol H 2 2. 48 mol H 2 6 mol HBr • 2. 48 mol H 2 are produced HBr is the limiting reactant and Al is the excess reactant

Limiting Reagent Practice • Example 11: How many grams of aluminum sulfate are produced if 23. 33 g Al reacts with 74. 44 g Cu. SO 4? • What reactant limits the reaction? Which reactant will not be complete used up? Al (s) + Cu. SO 4 (aq) Al 2(SO 4)3 (aq) + Cu (s)

Theoretical, Actual, and Percent Yield Theoretical Yield is how much product will be synthesized in ideal conditions. The theoretical yield is determined by finding the limiting reagent and using the mole ratio from a chemical equation to determine how much product can be made (Basically how much product should be produced) Actual Yield is how much product was actually synthesized in an experiment. This number is an experimental value. Percent Yield: is the ratio between the actual yield and theoretical yield multiplied by 100%. It indicates efficiency. Percentage Yield = Mass of Actual Yield x 100% Mass of Theoretical Yield

Theoretical Yield Example 12: What is the % yield of H 2 O if 138 g H 2 O is produced from 16 g H 2 and excess O 2? Step 1: write the balanced chemical equation 2 H 2 + O 2 2 H 2 O Step 2: determine actual and theoretical yield. Actual is given, theoretical is calculated: 1 mol H 22 mol H 2 O 18. 02 g # g H 2 O= 16 g H 2 x x x = 143 H 2 O 2. 02 g 2 mol 1 mol g H 2 yield H 2 O Step 3: Calculate % actual 138 g H 2 O % yield = x 100%= 96. 7 theoretic 143 g % al HO
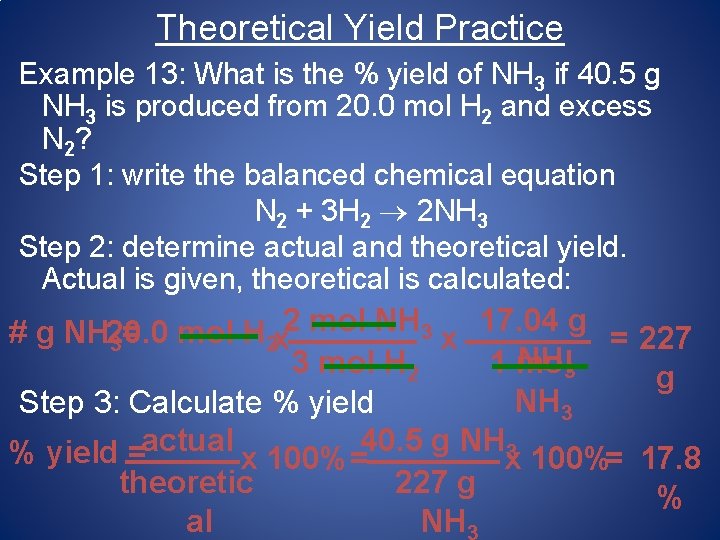
Theoretical Yield Practice Example 13: What is the % yield of NH 3 if 40. 5 g NH 3 is produced from 20. 0 mol H 2 and excess N 2? Step 1: write the balanced chemical equation N 2 + 3 H 2 2 NH 3 Step 2: determine actual and theoretical yield. Actual is given, theoretical is calculated: 2 mol NH 3 17. 04 g # g NH 20. 0 mol H = x = 227 3 2 x NH 3 3 mol H 2 1 mol g NH 3 Step 3: Calculate % yield actual 40. 5 g NH 3 % yield = x 100%= 17. 8 theoretic 227 g % al NH 3
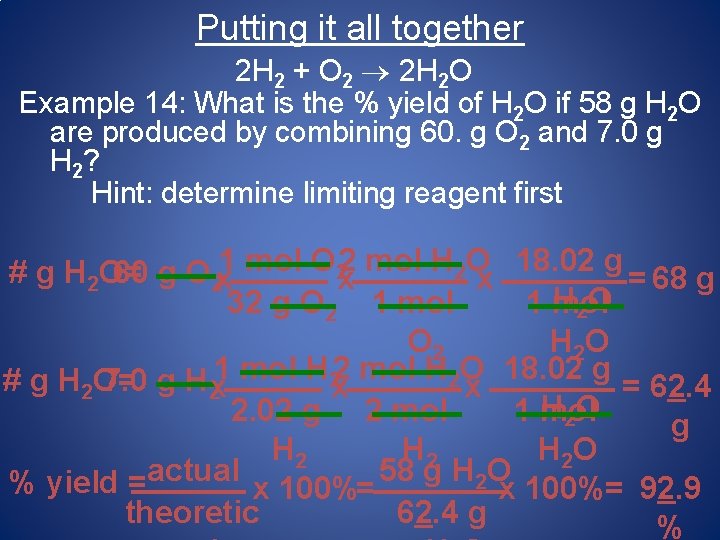
Putting it all together 2 H 2 + O 2 2 H 2 O Example 14: What is the % yield of H 2 O if 58 g H 2 O are produced by combining 60. g O 2 and 7. 0 g H 2? Hint: determine limiting reagent first 1 mol O 22 mol H 2 O 18. 02 g # g H 2 O= 60 g O 2 x x x = 68 g H 2 O 32 g O 2 1 mol O 2 H 2 O 1 mol H 22 mol H 2 O 18. 02 g # g H 2 O= 7. 0 g H 2 x x x = 62. 4 H 2 O 2. 02 g 2 mol 1 mol g H 2 H 2 O actual 58 g H 2 O % yield = x 100%= 92. 9 theoretic 62. 4 g %
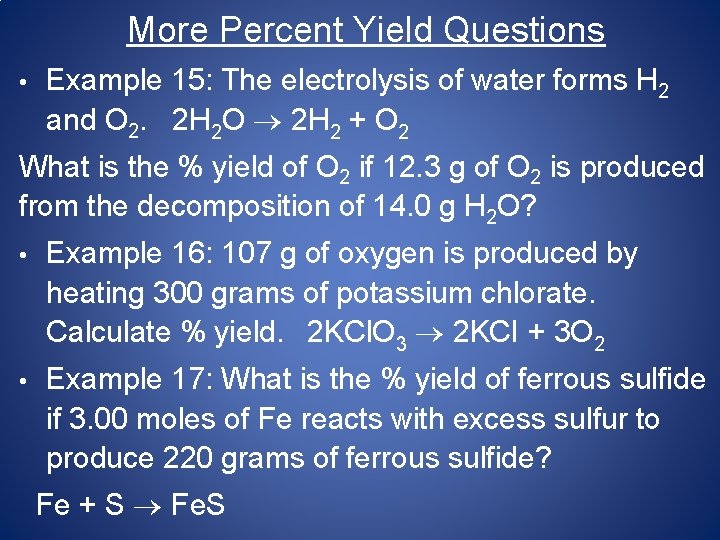
More Percent Yield Questions • Example 15: The electrolysis of water forms H 2 and O 2. 2 H 2 O 2 H 2 + O 2 What is the % yield of O 2 if 12. 3 g of O 2 is produced from the decomposition of 14. 0 g H 2 O? • Example 16: 107 g of oxygen is produced by heating 300 grams of potassium chlorate. Calculate % yield. 2 KCl. O 3 2 KCI + 3 O 2 • Example 17: What is the % yield of ferrous sulfide if 3. 00 moles of Fe reacts with excess sulfur to produce 220 grams of ferrous sulfide? Fe + S Fe. S
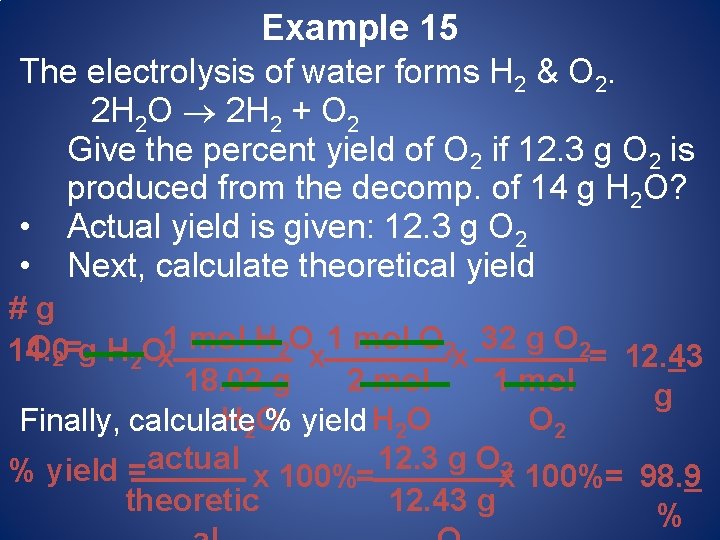
Example 15 The electrolysis of water forms H 2 & O 2. 2 H 2 O 2 H 2 + O 2 Give the percent yield of O 2 if 12. 3 g O 2 is produced from the decomp. of 14 g H 2 O? • Actual yield is given: 12. 3 g O 2 • Next, calculate theoretical yield # g 1 mol H 2 O 1 mol O 2 32 g O 2= 2 Ox 14. 0 g H x x = 12. 43 18. 02 g 2 mol 1 mol g H 2 O O 2 Finally, calculate % yield H 2 O actual 12. 3 g O 2 % yield = x 100%= 98. 9 theoretic 12. 43 g %
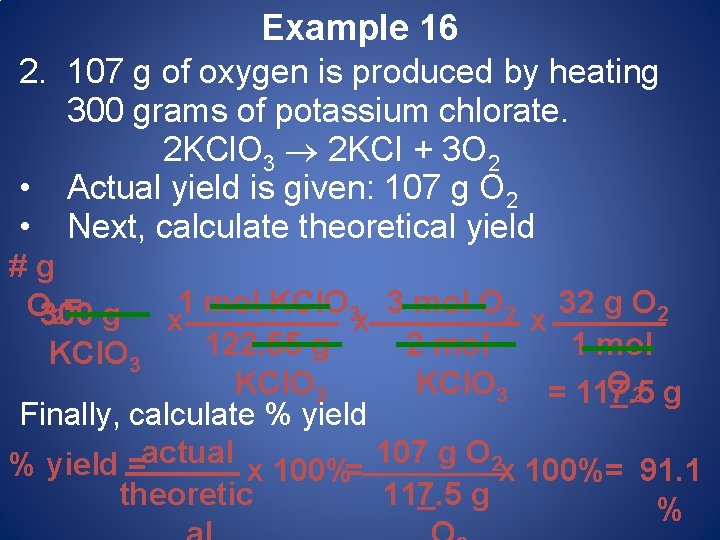
Example 16 2. 107 g of oxygen is produced by heating 300 grams of potassium chlorate. 2 KCl. O 3 2 KCI + 3 O 2 • Actual yield is given: 107 g O 2 • Next, calculate theoretical yield # g 1 mol KCl. O 3 3 mol O 2 32 g O 2 O 300 g 2= x x x 122. 55 g 2 mol 1 mol KCl. O 3 = 117. 5 g O 2 Finally, calculate % yield actual 107 g O 2 % yield = x 100%= 91. 1 theoretic 117. 5 g %

Example 17 3. What is % yield of ferrous sulfide if 3 mol Fe produce 220 grams of ferrous sulfide? Fe + S Fe. S • Actual yield is given: 220 g Fe. S • Next, calculate theoretical yield # g 3. 00 mol x 1 mol Fe. Sx 87. 91 g = 263. 7 g Fe. S 1 mol Fe. S= Fe Fe. S Finally, calculate % yield actual 220 g O 2 % yield = x 100%= 83. 4 theoretic 263. 7 g %
- Slides: 31