Stoichiometry Stoichiometry Steps 1 Balance the equation Is

Stoichiometry



Stoichiometry Steps 1. Balance the equation; Is it balanced?
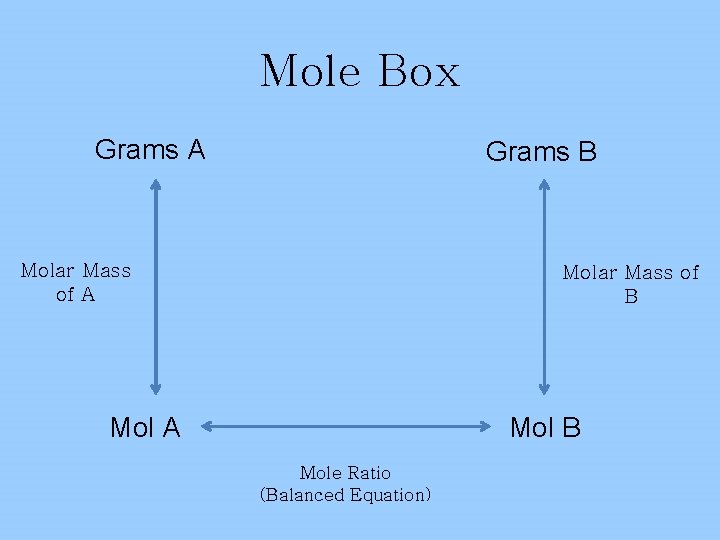
Mole Box Grams A Grams B Molar Mass of A Molar Mass of B Mol A Mol B Mole Ratio (Balanced Equation)
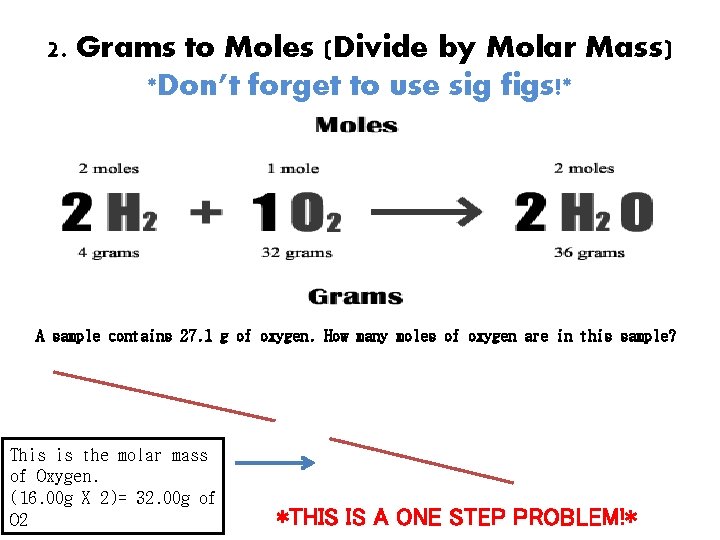
2. Grams to Moles (Divide by Molar Mass) *Don’t forget to use sig figs!* A sample contains 27. 1 g of oxygen. How many moles of oxygen are in this sample? 27. 1 grams of O 2 X 1 mole O 2 = 32 grams O 2 This is the molar mass of Oxygen. (16. 00 g X 2)= 32. 00 g of O 2 0. 847 moles of O 2 *THIS IS A ONE STEP PROBLEM!*

3. Moles to Moles ( Multiply by Mole Ratio) If you started with 3. 50 mol of hydrogen, how many moles of oxygen could be formed? 3. 50 mol H 2 X 1 mole O 2 = 1. 75 moles of O 2 2 moles H 2

4. Mole to Grams (Multiply by Molar Mass) If you needed to produce 12. 2 g of water, how many grams of hydrogen would you need? 12. 2 moles of H 2 O X 2 moles H 2 X 2 grams H 2 = 24. 4 grams of H 2 2 moles H 2 O 1 mole H 2 *THIS IS A TWO STEP PROBLEM!*
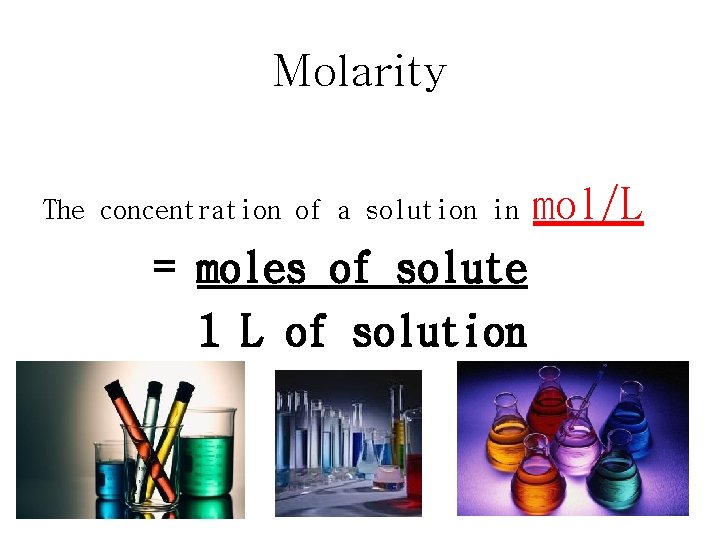
Molarity The concentration of a solution in = moles of solute 1 L of solution mol/L

Finding Moles of a Solution Use the formula; cv = n Concentration(mol/L) X Volume(L) = Moles(mol)
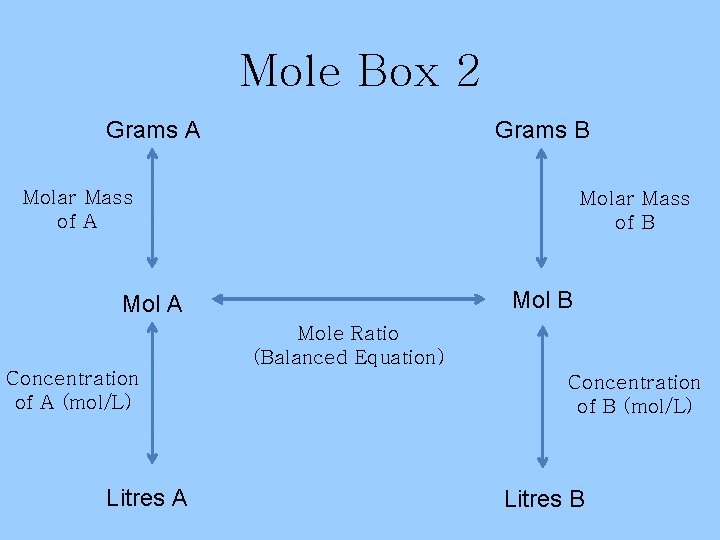
Mole Box 2 Grams B Grams A Molar Mass of B Mol A Mole Ratio (Balanced Equation) Concentration of A (mol/L) Litres A Concentration of B (mol/L) Litres B
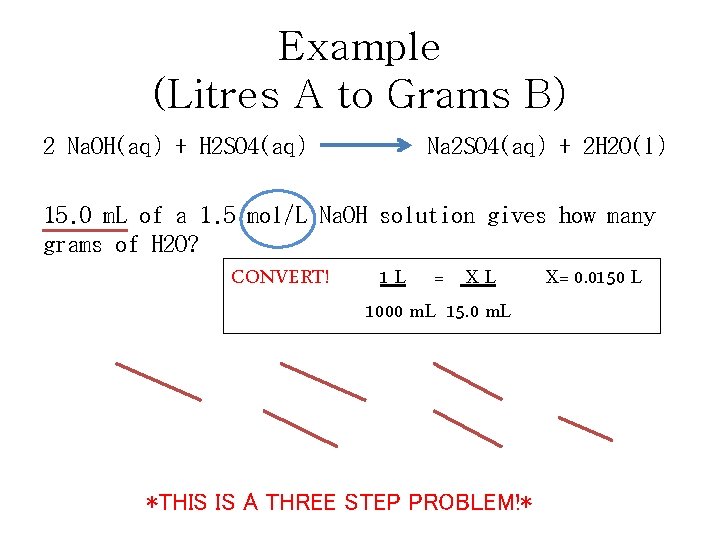
Example (Litres A to Grams B) 2 Na. OH(aq) + H 2 SO 4(aq) Na 2 SO 4(aq) + 2 H 2 O(l) 15. 0 m. L of a 1. 5 mol/L Na. OH solution gives how many grams of H 2 O? CONVERT! 1 L = XL 1000 m. L 15. 0 m. L X= 0. 0150 L Na. OH X 1. 5 mol Na. OH X 2 mol H 2 O X 18 g H 2 O = 0. 405 g 1 L Na. OH 2 mol Na. OH 1 mol H 2 O *THIS IS A THREE STEP PROBLEM!*
Stoichiometry with Gases The stoichiometry of gases is based on the ratio between the quantities of gas involved in a chemical reaction. This method is used to predict the quantity of a reactant or product involved in a chemical reaction in which one of the compounds is a gas.
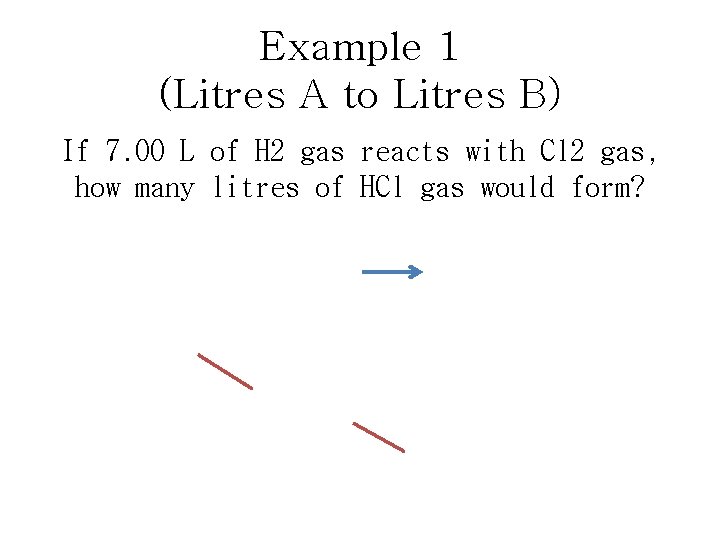
Example 1 (Litres A to Litres B) If 7. 00 L of H 2 gas reacts with Cl 2 gas, how many litres of HCl gas would form? H 2 + Cl 2 2 HCl 7. 00 L H 2 X 2 L HCl = 14. 0 L HCl 1 L H 2
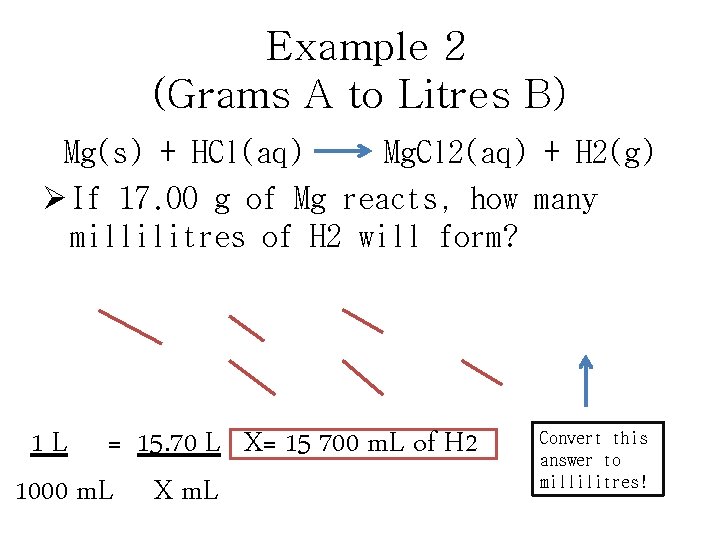
Example 2 (Grams A to Litres B) Mg(s) + HCl(aq) Mg. Cl 2(aq) + H 2(g) Ø If 17. 00 g of Mg reacts, how many millilitres of H 2 will form? 17. 00 g Mg X 1 mol H 2 X 22. 4 L H 2 = 15. 70 L of H 2 24. 31 g Mg 1 mol H 2 1 L = 15. 70 L X= 15 700 m. L of H 2 1000 m. L X m. L Convert this answer to millilitres!

Classifying Reactions 1. Combustion= Burning with Oxygen Tips: Ø If C is in the reactants, CO 2 will be a product. Example: • C + O 2 CO 2 Ø If H 2 is in the reactants, H 2 O will be a product. Example: • 2 H 2 + O 2 2 H 2 O • CH 4 + 2 O 2 CO 2 + 2 H 2 O
2. Synthesis Simpler reactants combine to make more complex products. Examples: • Mg + Cl 2 • CO 2 + H 2 O Mg. Cl 2 H 2 CO 3
3. Decomposition is when complex Reactants break down to become simpler molecules. Examples: • H 2 CO 3 • BF 3 NH 3 H 2 O + CO 2 BF 3 + NH 3
4. Single Displacement An element and a compound are required for single displacement. The element in the reactants will “replace” or “displace” one of the elements in the compound. *It is important to remember that metals replace metals only, and non-metals replace non-metals only. Examples: • A + B B + AC • Na(s) + H 2 O(l) Na. OH(aq) + H 2(g)
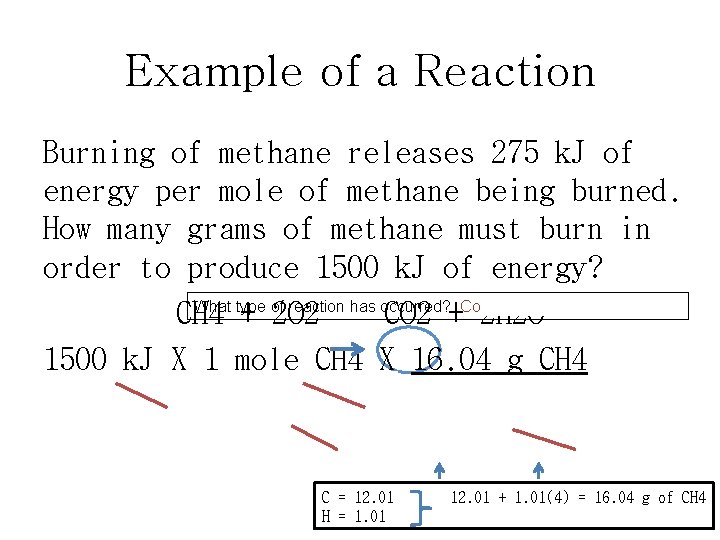
Example of a Reaction Burning of methane releases 275 k. J of energy per mole of methane being burned. How many grams of methane must burn in order to produce 1500 k. J of energy? What type of reaction has occurred? Combustion reaction. CH 4 + 2 O 2 CO 2 + 2 H 2 O 1500 k. J X 1 mole CH 4 X 16. 04 g CH 4 275 k. J 1 mole CH 4 = 88 g of CH 4 C = 12. 01 H = 1. 01 12. 01 + 1. 01(4) = 16. 04 g of CH 4

Bibliography • Couture, Ivan, Marie-Eve Lacombe-Harvey, and Genevieve Levasseur-Thériault. Quantum Chemistry Student Textbook. Ed. Marie-Eve Robitaille, Isabel Rusin, and Colleen Ovenden. Trans. Jacquie Charlton, Cristina Cusano, Natasha De. Cruz, Joann Egar, and Gwen Schulman. Montreal: Cheneliere Education, 2011. Print. • Doty, Wendy L. "Stoichiometry Notes. " Downey Unified School District. N. p. , 2006. Web. 10 Oct. 2012. <http: //www. dusd. net/staff/rcramm/Chemistry/Unit_8/ Stoichiometery%20 Notes. pdf>.
- Slides: 21