Stoichiometry S Composition S Mass relationships in compounds


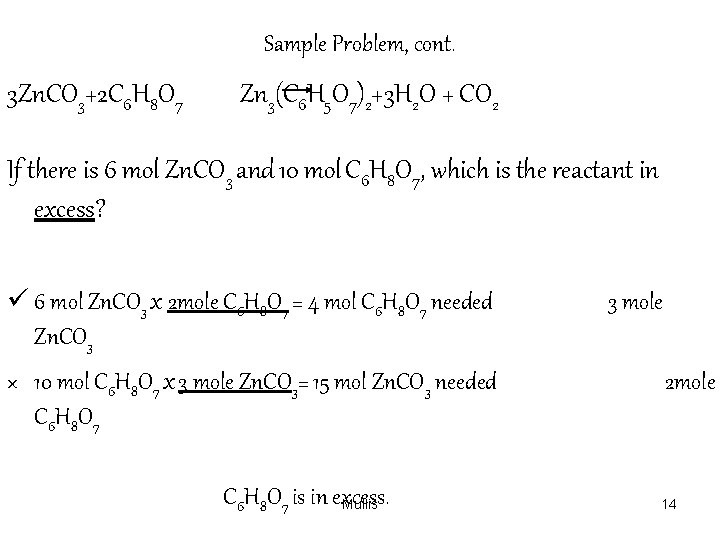
- Slides: 18

Stoichiometry (S) • Composition S: Mass relationships in compounds • Reaction S: Mass relationships between reactants and products • To find amounts of products and/or reactants, you must convert both to moles! Mullis 1

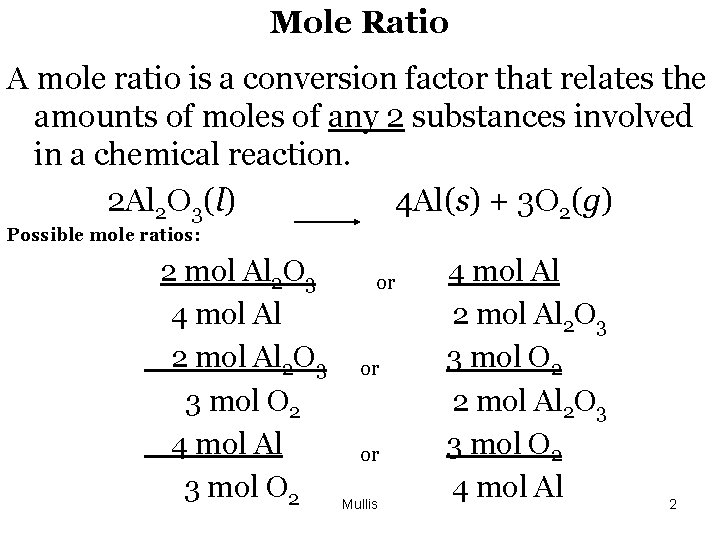
Mole Ratio A mole ratio is a conversion factor that relates the amounts of moles of any 2 substances involved in a chemical reaction. 2 Al 2 O 3(l) 4 Al(s) + 3 O 2(g) Possible mole ratios: 2 mol Al 2 O 3 4 mol Al 2 O 3 3 mol O 2 4 mol Al 3 mol O 2 or or or Mullis 4 mol Al 2 O 3 3 mol O 2 2 mol Al 2 O 3 3 mol O 2 4 mol Al 2

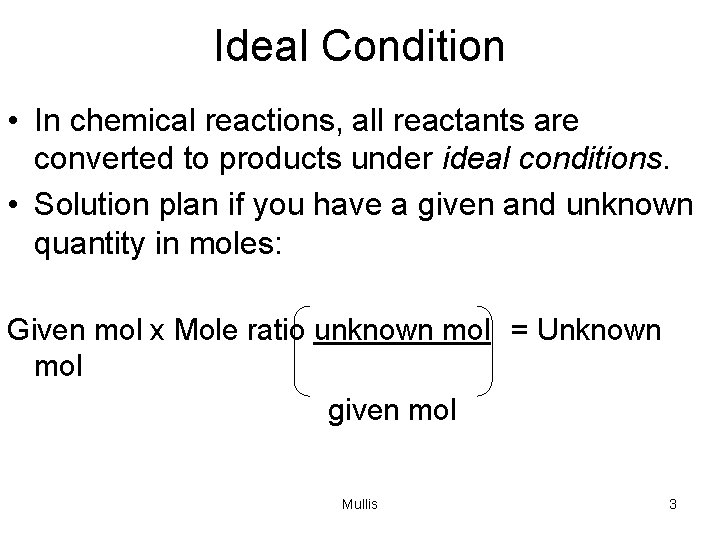
Ideal Condition • In chemical reactions, all reactants are converted to products under ideal conditions. • Solution plan if you have a given and unknown quantity in moles: Given mol x Mole ratio unknown mol = Unknown mol given mol Mullis 3

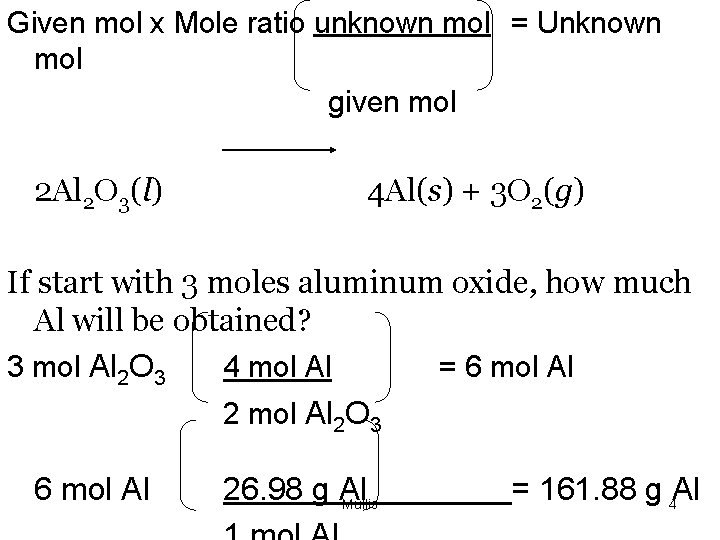
Given mol x Mole ratio unknown mol = Unknown mol given mol 2 Al 2 O 3(l) 4 Al(s) + 3 O 2(g) If start with 3 moles aluminum oxide, how much Al will be obtained? 3 mol Al 2 O 3 4 mol Al = 6 mol Al 2 O 3 6 mol Al 26. 98 g Al Mullis = 161. 88 g 4 Al

Conversion Reminders Mullis 5

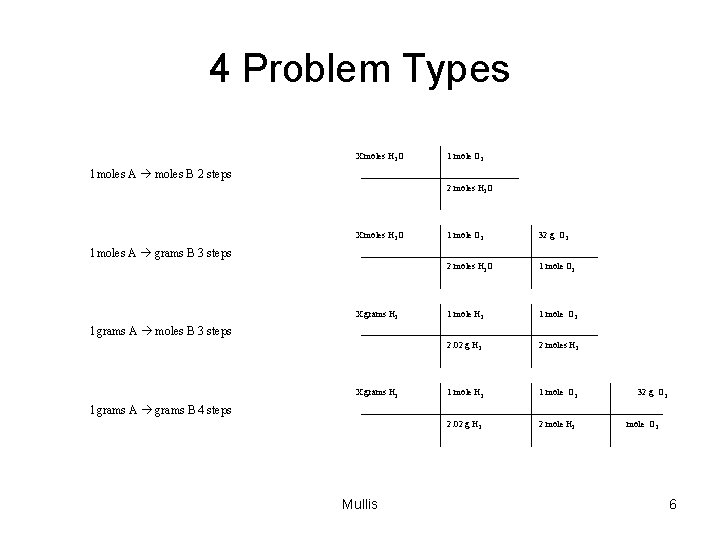
4 Problem Types X moles H 2 O 1 mole O 2 1 moles A moles B 2 steps 2 moles H 2 O X moles H 2 O 1 mole O 2 32 g O 2 2 moles H 2 O 1 mole O 2 1 mole H 2 1 mole O 2 2. 02 g H 2 2 moles H 2 1 mole O 2 2. 02 g H 2 2 mole H 2 1 moles A grams B 3 steps X grams H 2 1 grams A moles B 3 steps X grams H 2 32 g O 2 1 grams A grams B 4 steps Mullis 1 mole O 2 6

Practice Problems 1. When sodium azide (Na. N 3) is activated in an automobile airbag, nitrogen gas and sodium are produced. If 0. 500 mol Na. N 3 react, what mass in grams of nitrogen would result? 2. Carborundum, Si. C, is a hard substance made by combining silicon dioxide with coke (C). The products are Si. C and CO. What is the mass of Si. C in grams from the complete reaction of 7 2. 00 mol carbon? Mullis

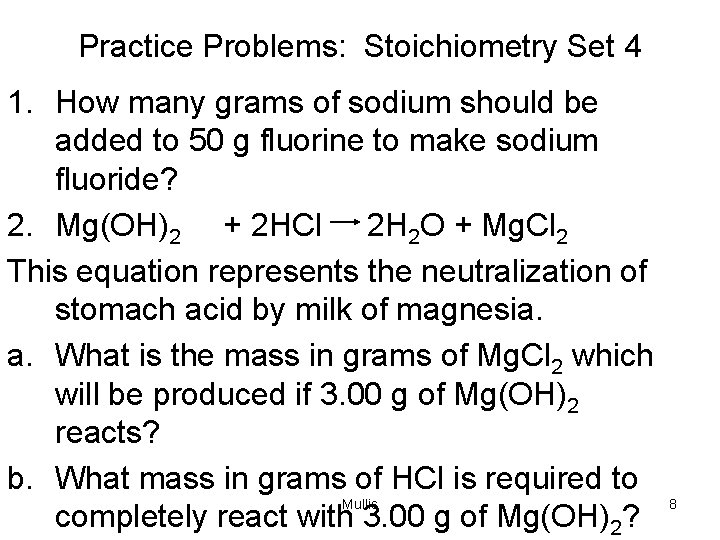
Practice Problems: Stoichiometry Set 4 1. How many grams of sodium should be added to 50 g fluorine to make sodium fluoride? 2. Mg(OH)2 + 2 HCl 2 H 2 O + Mg. Cl 2 This equation represents the neutralization of stomach acid by milk of magnesia. a. What is the mass in grams of Mg. Cl 2 which will be produced if 3. 00 g of Mg(OH)2 reacts? b. What mass in grams of HCl is required to Mullis completely react with 3. 00 g of Mg(OH)2? 8

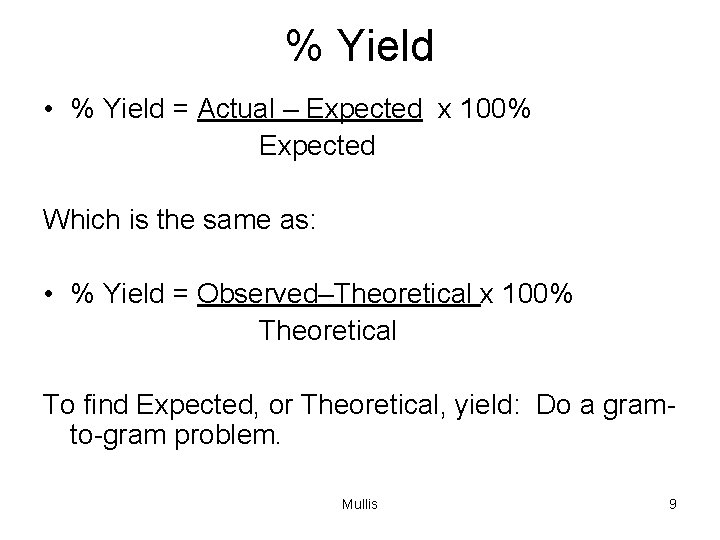
% Yield • % Yield = Actual – Expected x 100% Expected Which is the same as: • % Yield = Observed–Theoretical x 100% Theoretical To find Expected, or Theoretical, yield: Do a gramto-gram problem. Mullis 9

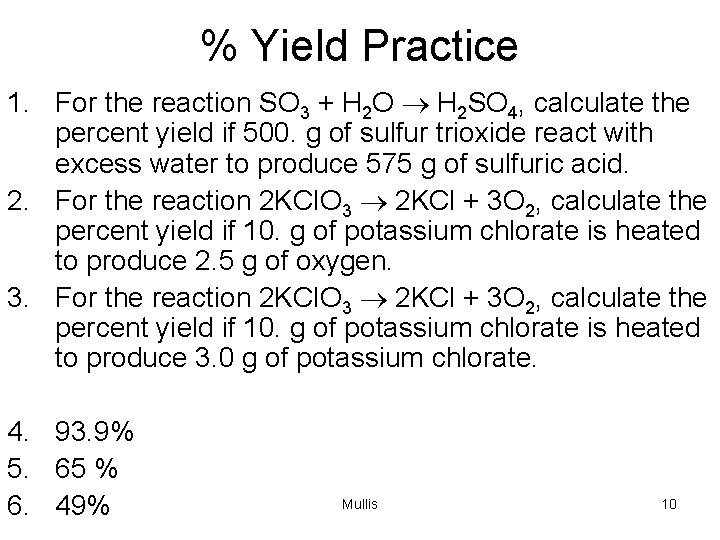
% Yield Practice 1. For the reaction SO 3 + H 2 O ® H 2 SO 4, calculate the percent yield if 500. g of sulfur trioxide react with excess water to produce 575 g of sulfuric acid. 2. For the reaction 2 KCl. O 3 ® 2 KCl + 3 O 2, calculate the percent yield if 10. g of potassium chlorate is heated to produce 2. 5 g of oxygen. 3. For the reaction 2 KCl. O 3 ® 2 KCl + 3 O 2, calculate the percent yield if 10. g of potassium chlorate is heated to produce 3. 0 g of potassium chlorate. 4. 93. 9% 5. 65 % 6. 49% Mullis 10

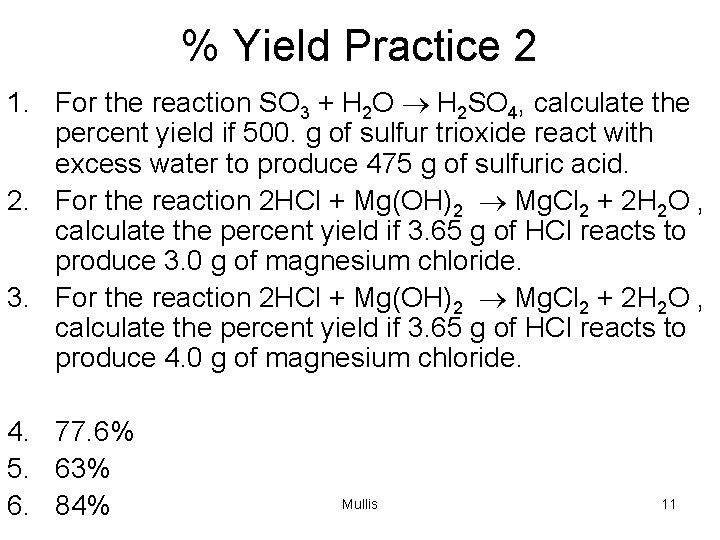
% Yield Practice 2 1. For the reaction SO 3 + H 2 O ® H 2 SO 4, calculate the percent yield if 500. g of sulfur trioxide react with excess water to produce 475 g of sulfuric acid. 2. For the reaction 2 HCl + Mg(OH)2 ® Mg. Cl 2 + 2 H 2 O , calculate the percent yield if 3. 65 g of HCl reacts to produce 3. 0 g of magnesium chloride. 3. For the reaction 2 HCl + Mg(OH)2 ® Mg. Cl 2 + 2 H 2 O , calculate the percent yield if 3. 65 g of HCl reacts to produce 4. 0 g of magnesium chloride. 4. 77. 6% 5. 63% 6. 84% Mullis 11

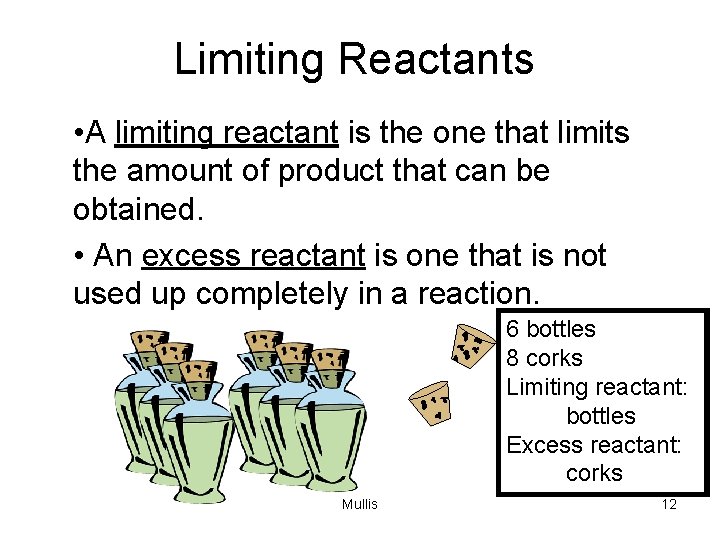
Limiting Reactants • A limiting reactant is the one that limits the amount of product that can be obtained. • An excess reactant is one that is not used up completely in a reaction. 6 bottles 8 corks Limiting reactant: bottles Excess reactant: corks Mullis 12

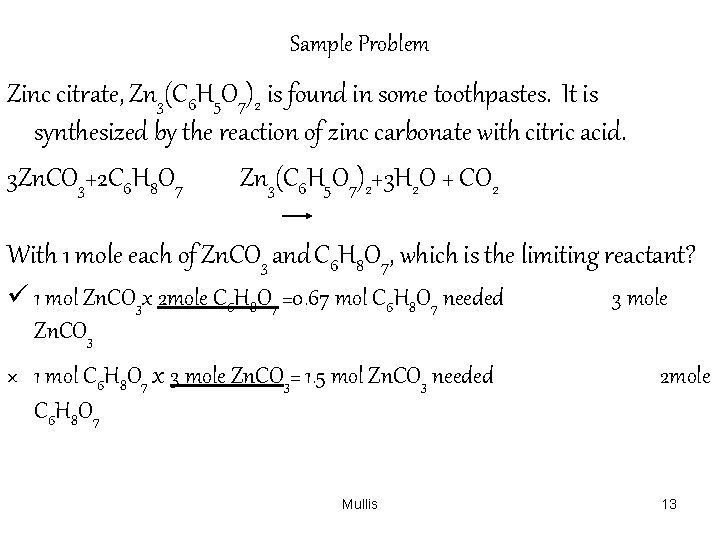
Sample Problem Zinc citrate, Zn 3(C 6 H 5 O 7)2 is found in some toothpastes. It is synthesized by the reaction of zinc carbonate with citric acid. 3 Zn. CO 3+2 C 6 H 8 O 7 Zn 3(C 6 H 5 O 7)2+3 H 2 O + CO 2 With 1 mole each of Zn. CO 3 and C 6 H 8 O 7, which is the limiting reactant? ü 1 mol Zn. CO 3 x 2 mole C 6 H 8 O 7 =0. 67 mol C 6 H 8 O 7 needed Zn. CO 3 × 1 mol C 6 H 8 O 7 x 3 mole Zn. CO 3= 1. 5 mol Zn. CO 3 needed C 6 H 8 O 7 Mullis 3 mole 2 mole 13
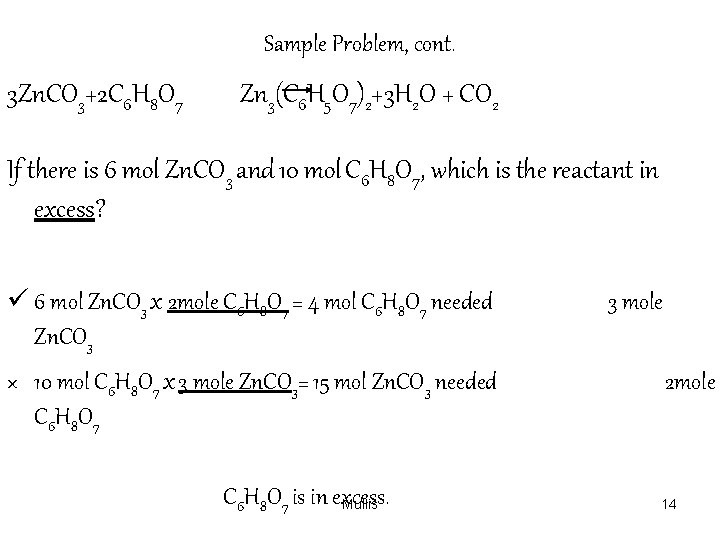
Sample Problem, cont. 3 Zn. CO 3+2 C 6 H 8 O 7 Zn 3(C 6 H 5 O 7)2+3 H 2 O + CO 2 If there is 6 mol Zn. CO 3 and 10 mol C 6 H 8 O 7, which is the reactant in excess? ü 6 mol Zn. CO 3 x 2 mole C 6 H 8 O 7 = 4 mol C 6 H 8 O 7 needed Zn. CO 3 × 10 mol C 6 H 8 O 7 x 3 mole Zn. CO 3= 15 mol Zn. CO 3 needed C 6 H 8 O 7 C 6 H 8 O 7 is in excess. Mullis 3 mole 2 mole 14

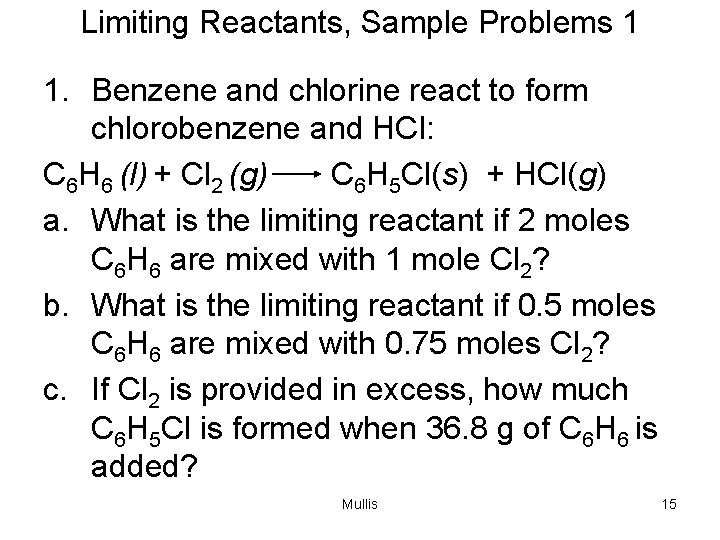
Limiting Reactants, Sample Problems 1 1. Benzene and chlorine react to form chlorobenzene and HCl: C 6 H 6 (l) + Cl 2 (g) C 6 H 5 Cl(s) + HCl(g) a. What is the limiting reactant if 2 moles C 6 H 6 are mixed with 1 mole Cl 2? b. What is the limiting reactant if 0. 5 moles C 6 H 6 are mixed with 0. 75 moles Cl 2? c. If Cl 2 is provided in excess, how much C 6 H 5 Cl is formed when 36. 8 g of C 6 H 6 is added? Mullis 15

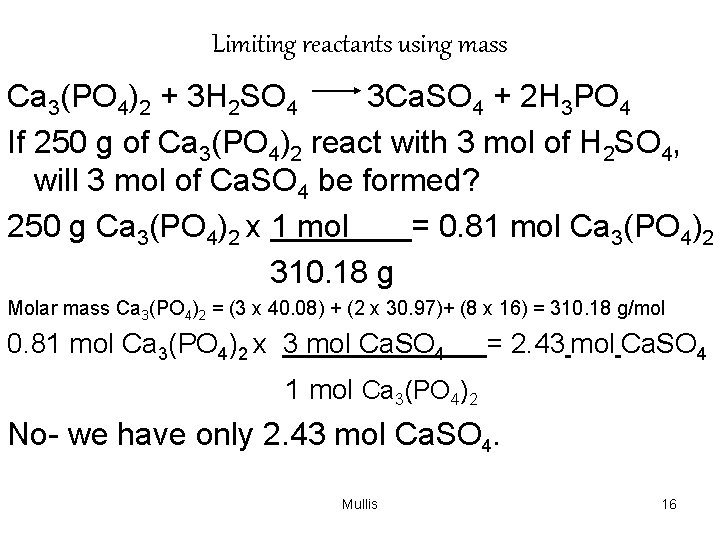
Limiting reactants using mass Ca 3(PO 4)2 + 3 H 2 SO 4 3 Ca. SO 4 + 2 H 3 PO 4 If 250 g of Ca 3(PO 4)2 react with 3 mol of H 2 SO 4, will 3 mol of Ca. SO 4 be formed? 250 g Ca 3(PO 4)2 x 1 mol = 0. 81 mol Ca 3(PO 4)2 310. 18 g Molar mass Ca 3(PO 4)2 = (3 x 40. 08) + (2 x 30. 97)+ (8 x 16) = 310. 18 g/mol 0. 81 mol Ca 3(PO 4)2 x 3 mol Ca. SO 4 = 2. 43 mol Ca. SO 4 1 mol Ca 3(PO 4)2 No- we have only 2. 43 mol Ca. SO 4. Mullis 16

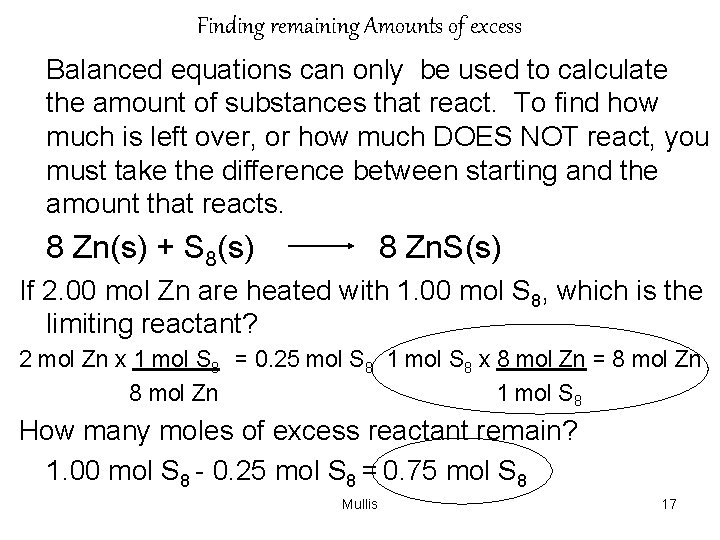
Finding remaining Amounts of excess Balanced equations can only be used to calculate the amount of substances that react. To find how much is left over, or how much DOES NOT react, you must take the difference between starting and the amount that reacts. 8 Zn(s) + S 8(s) 8 Zn. S(s) If 2. 00 mol Zn are heated with 1. 00 mol S 8, which is the limiting reactant? 2 mol Zn x 1 mol S 8 = 0. 25 mol S 8 1 mol S 8 x 8 mol Zn = 8 mol Zn 1 mol S 8 How many moles of excess reactant remain? 1. 00 mol S 8 - 0. 25 mol S 8 = 0. 75 mol S 8 Mullis 17

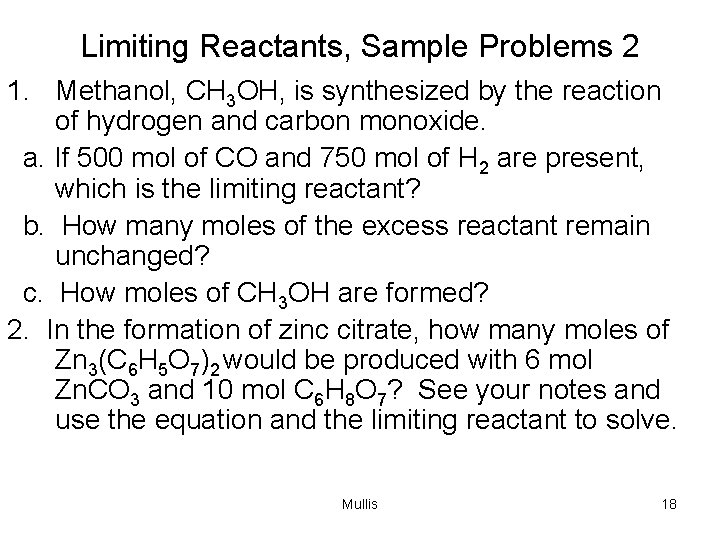
Limiting Reactants, Sample Problems 2 1. Methanol, CH 3 OH, is synthesized by the reaction of hydrogen and carbon monoxide. a. If 500 mol of CO and 750 mol of H 2 are present, which is the limiting reactant? b. How many moles of the excess reactant remain unchanged? c. How moles of CH 3 OH are formed? 2. In the formation of zinc citrate, how many moles of Zn 3(C 6 H 5 O 7)2 would be produced with 6 mol Zn. CO 3 and 10 mol C 6 H 8 O 7? See your notes and use the equation and the limiting reactant to solve. Mullis 18