Stoichiometry Quantitative relationships in a chemical equation CH

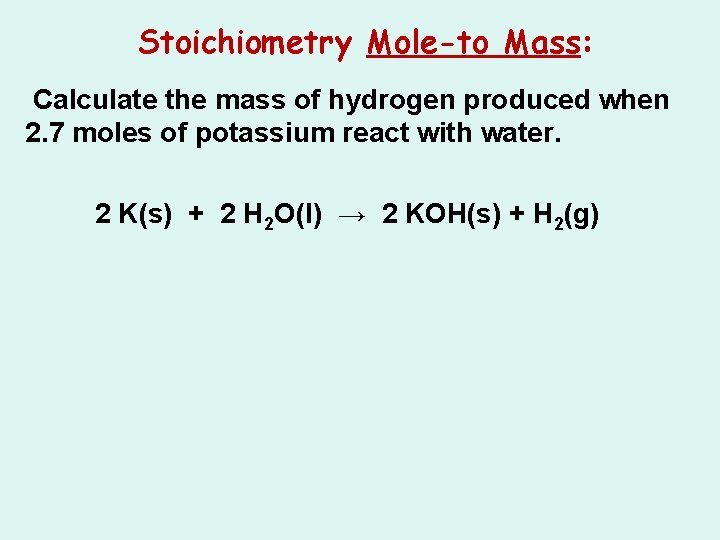
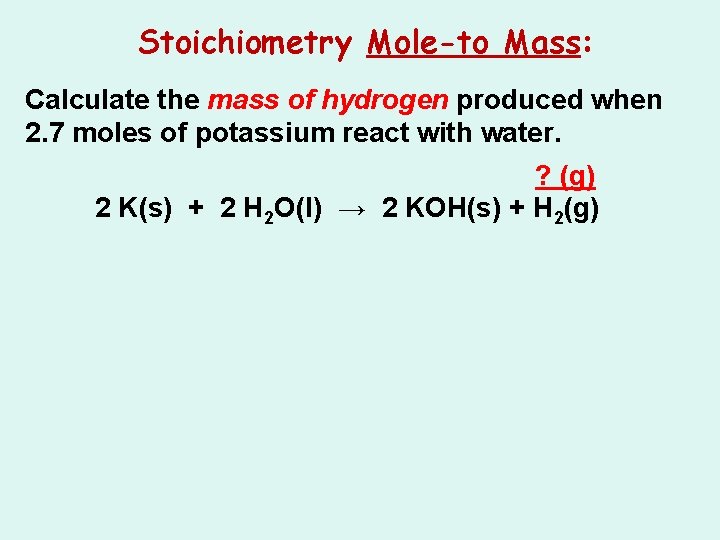
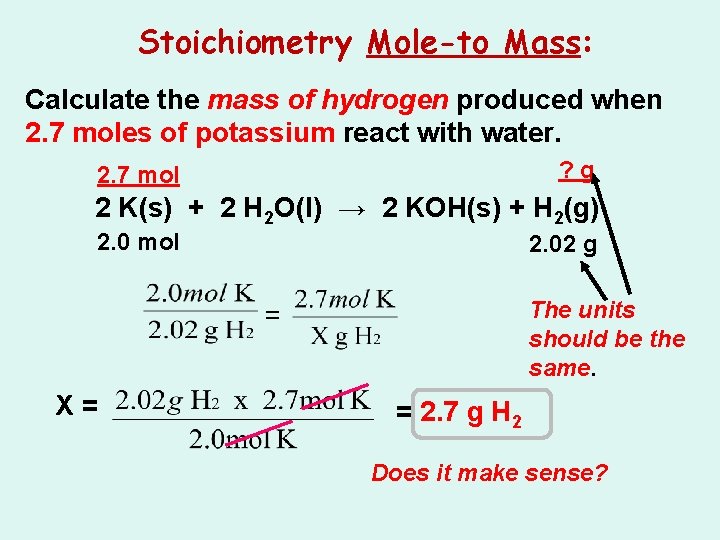

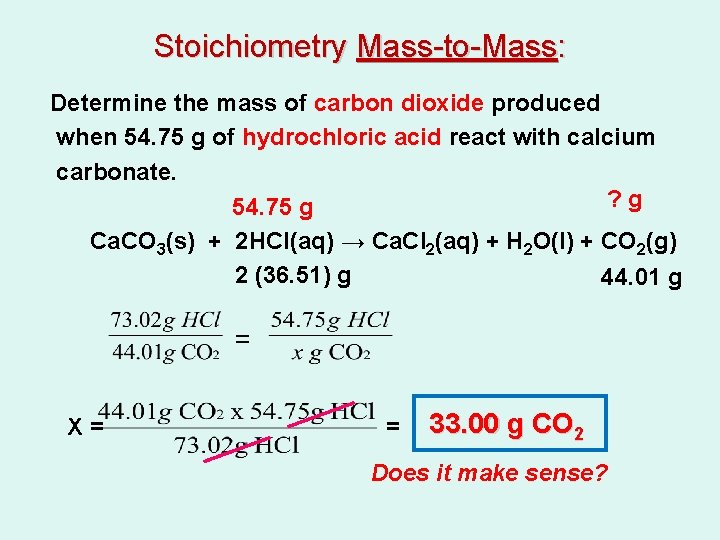
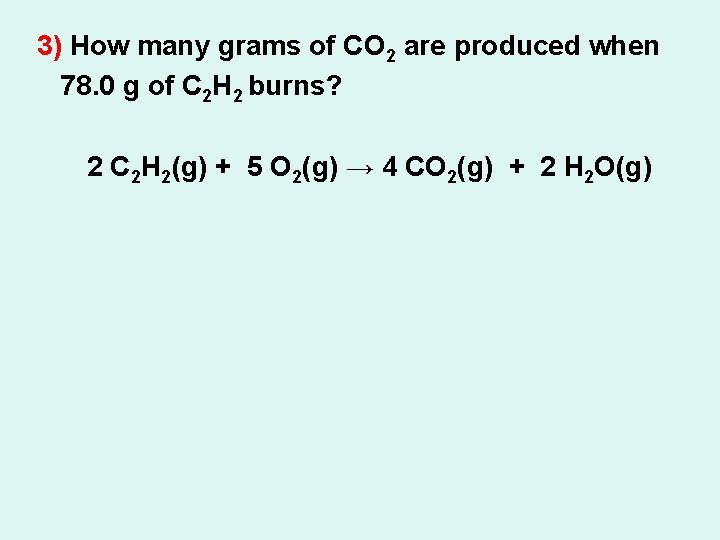

- Slides: 18

Stoichiometry: Quantitative relationships in a chemical equation

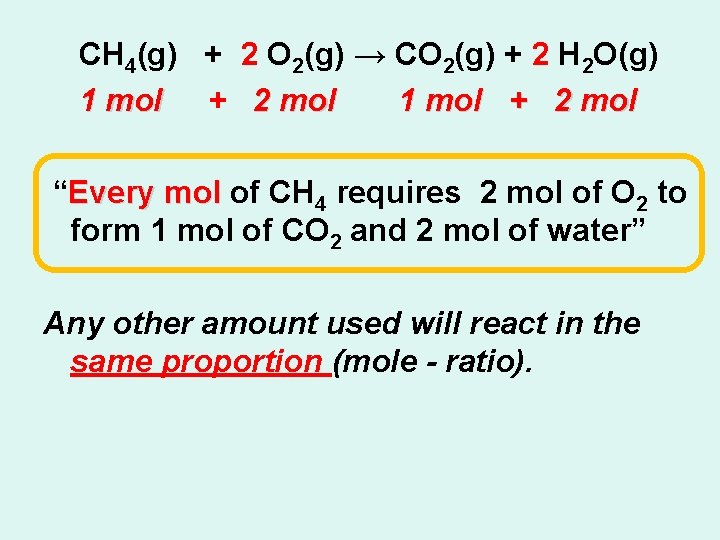
CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) 1 mol + 2 mol “Every mol of CH 4 requires 2 mol of O 2 to form 1 mol of CO 2 and 2 mol of water” Any other amount used will react in the same proportion (mole - ratio).

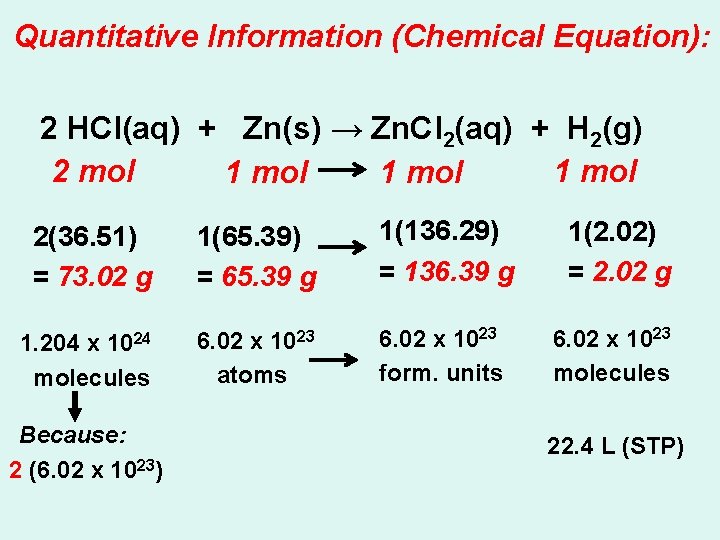
Quantitative Information (Chemical Equation): 2 HCl(aq) + Zn(s) → Zn. Cl 2(aq) + H 2(g) 2 mol 1 mol 2(36. 51) = 73. 02 g 1(65. 39) = 65. 39 g 1(136. 29) = 136. 39 g 1. 204 x 1024 molecules 6. 02 x 1023 atoms 6. 02 x 1023 form. units Because: 2 (6. 02 x 1023) 1(2. 02) = 2. 02 g 6. 02 x 1023 molecules 22. 4 L (STP)

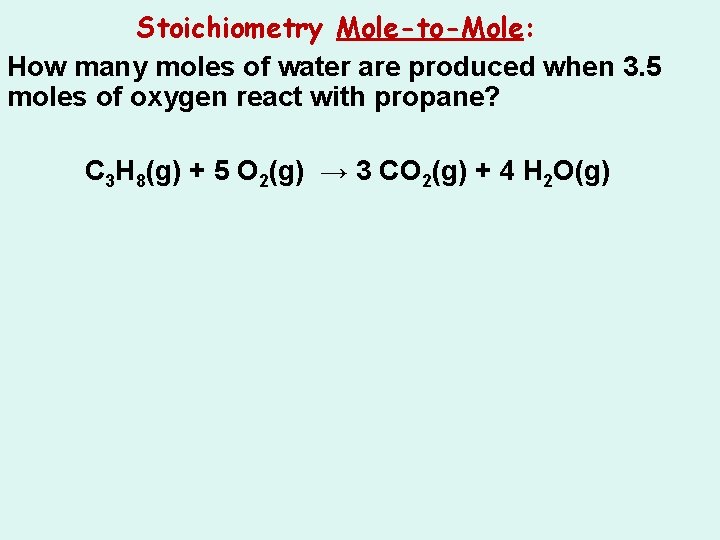
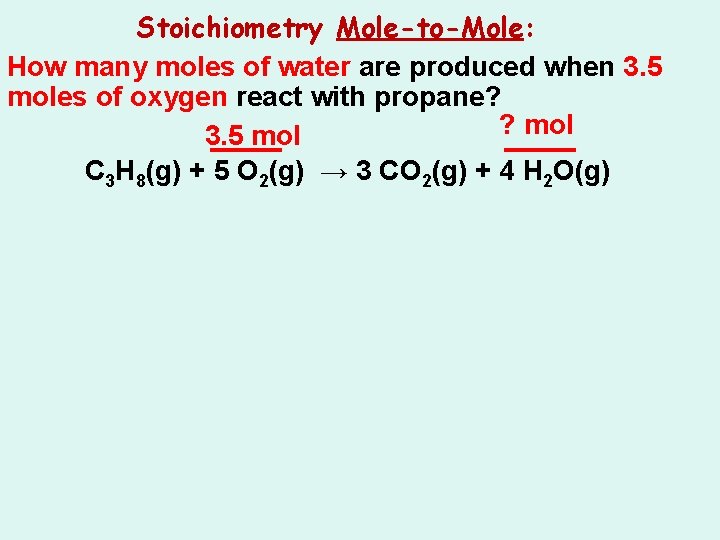
Stoichiometry Mole-to-Mole: How many moles of water are produced when 3. 5 moles of oxygen react with propane? C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g)

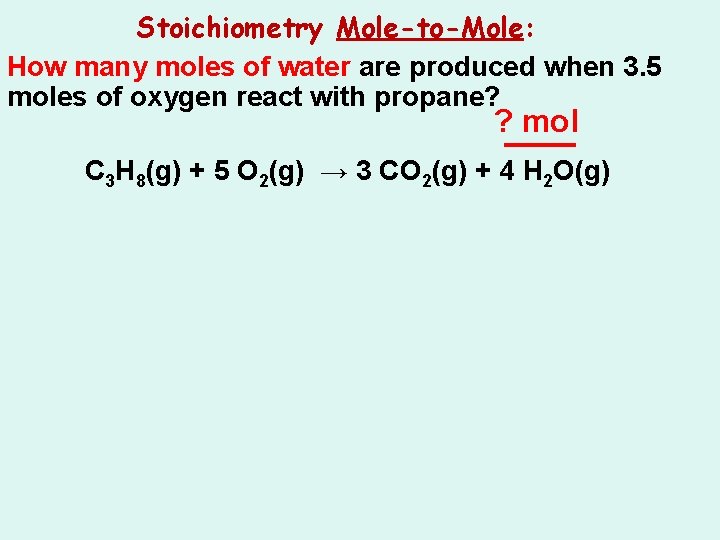
Stoichiometry Mole-to-Mole: How many moles of water are produced when 3. 5 moles of oxygen react with propane? ? mol C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g)

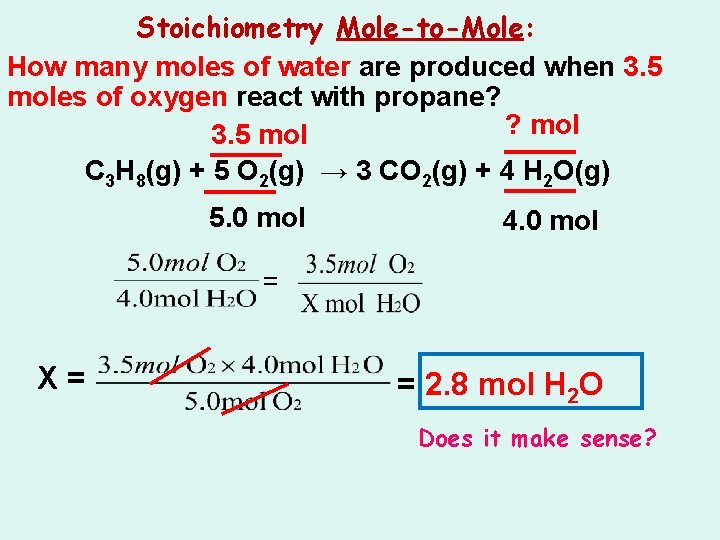
Stoichiometry Mole-to-Mole: How many moles of water are produced when 3. 5 moles of oxygen react with propane? ? mol 3. 5 mol C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g)

Stoichiometry Mole-to-Mole: How many moles of water are produced when 3. 5 moles of oxygen react with propane? ? mol 3. 5 mol C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) 5. 0 mol 4. 0 mol = X= = 2. 8 mol H 2 O Does it make sense?
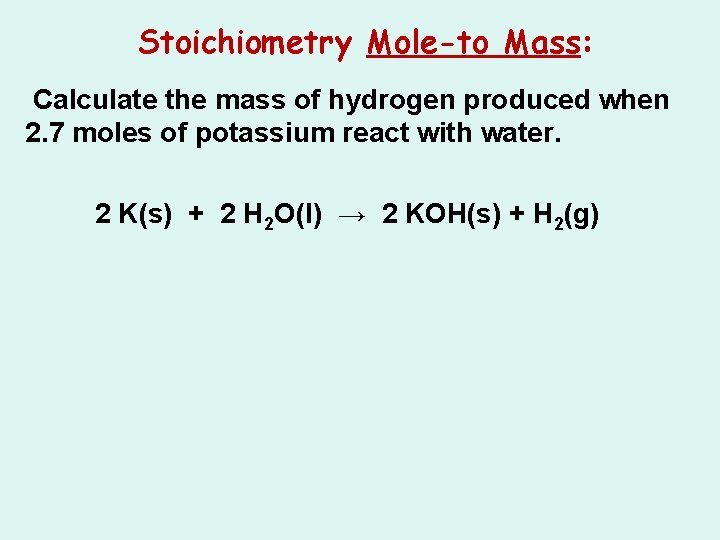
Stoichiometry Mole-to Mass: Calculate the mass of hydrogen produced when 2. 7 moles of potassium react with water. 2 K(s) + 2 H 2 O(l) → 2 KOH(s) + H 2(g)
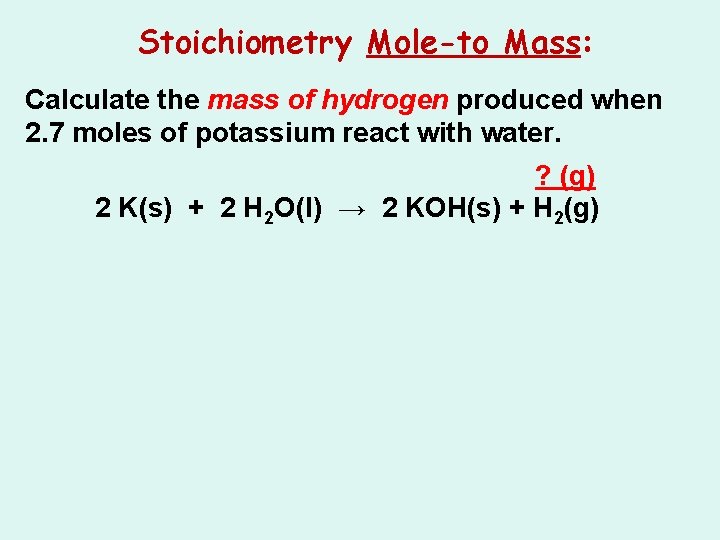
Stoichiometry Mole-to Mass: Calculate the mass of hydrogen produced when 2. 7 moles of potassium react with water. ? (g) 2 K(s) + 2 H 2 O(l) → 2 KOH(s) + H 2(g)
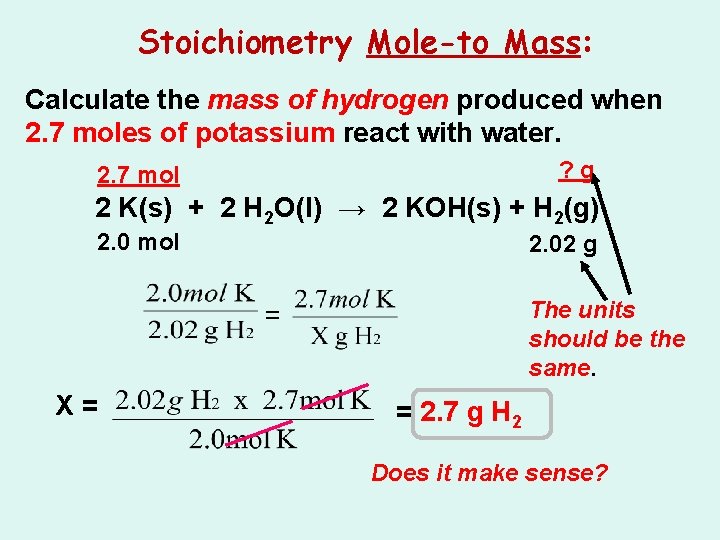
Stoichiometry Mole-to Mass: Calculate the mass of hydrogen produced when 2. 7 moles of potassium react with water. ? g 2. 7 mol 2 K(s) + 2 H 2 O(l) → 2 KOH(s) + H 2(g) 2. 0 mol 2. 02 g The units should be the same. = X= = 2. 7 g H 2 Does it make sense?

Stoichiometry Mass-to-Mass: Determine the mass of carbon dioxide produced when 54. 75 g of hydrochloric acid react with calcium carbonate. Ca. CO 3(s) + 2 HCl(aq) → Ca. Cl 2(aq) + H 2 O(l) + CO 2(g)
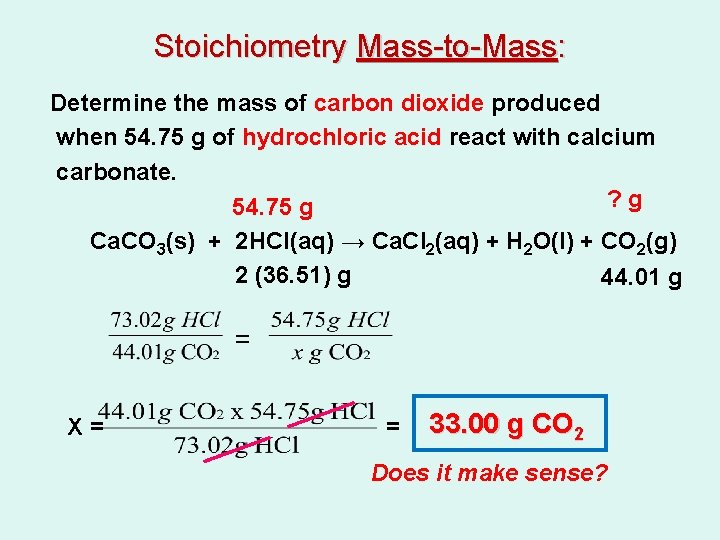
Stoichiometry Mass-to-Mass: Determine the mass of carbon dioxide produced when 54. 75 g of hydrochloric acid react with calcium carbonate. ? g 54. 75 g Ca. CO 3(s) + 2 HCl(aq) → Ca. Cl 2(aq) + H 2 O(l) + CO 2(g) 2 (36. 51) g 44. 01 g = X= = 33. 00 g CO 2 Does it make sense?

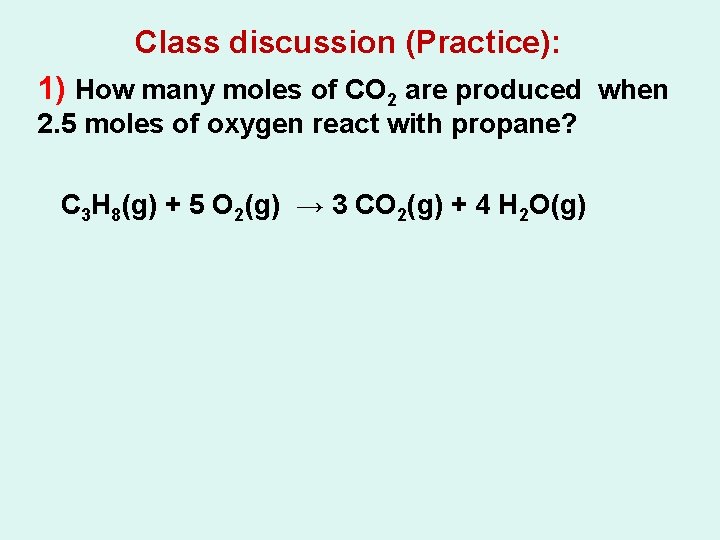
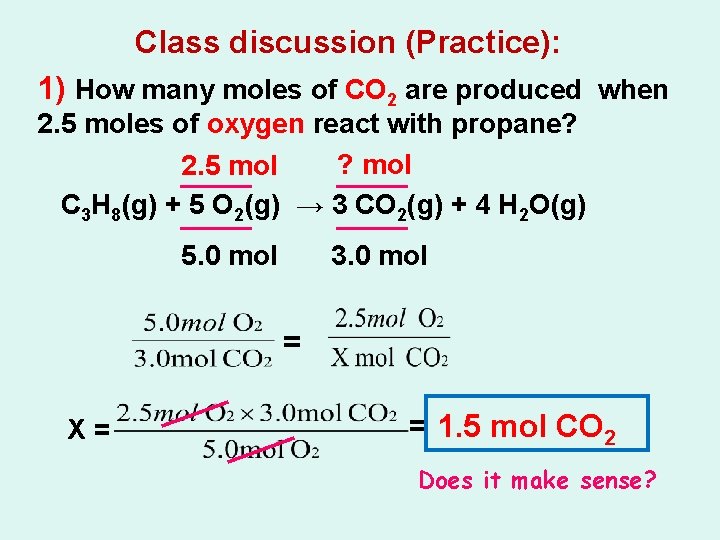
Class discussion (Practice): 1) How many moles of CO 2 are produced when 2. 5 moles of oxygen react with propane? C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g)

Class discussion (Practice): 1) How many moles of CO 2 are produced when 2. 5 moles of oxygen react with propane? ? mol 2. 5 mol C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) 5. 0 mol 3. 0 mol = X= = 1. 5 mol CO 2 Does it make sense?

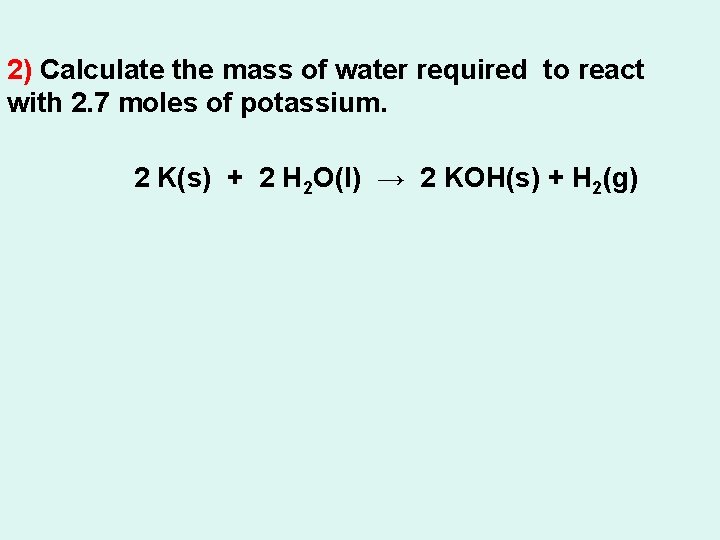
2) Calculate the mass of water required to react with 2. 7 moles of potassium. 2 K(s) + 2 H 2 O(l) → 2 KOH(s) + H 2(g)

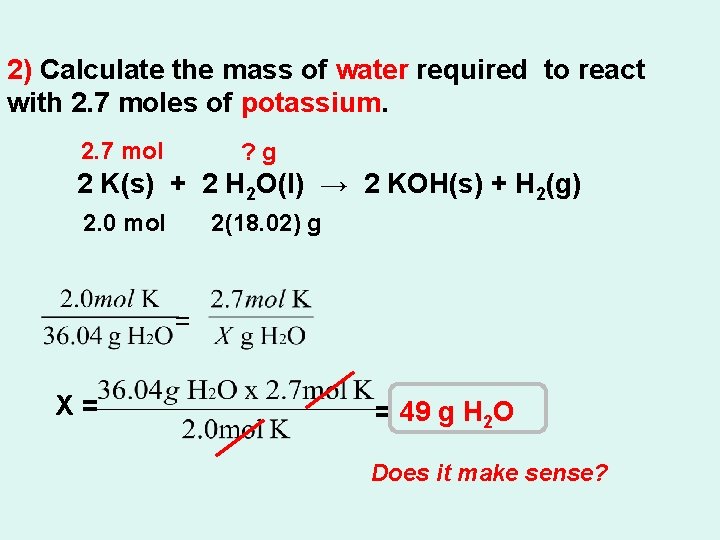
2) Calculate the mass of water required to react with 2. 7 moles of potassium. 2. 7 mol ? g 2 K(s) + 2 H 2 O(l) → 2 KOH(s) + H 2(g) 2. 0 mol 2(18. 02) g = X= = 49 g H 2 O Does it make sense?
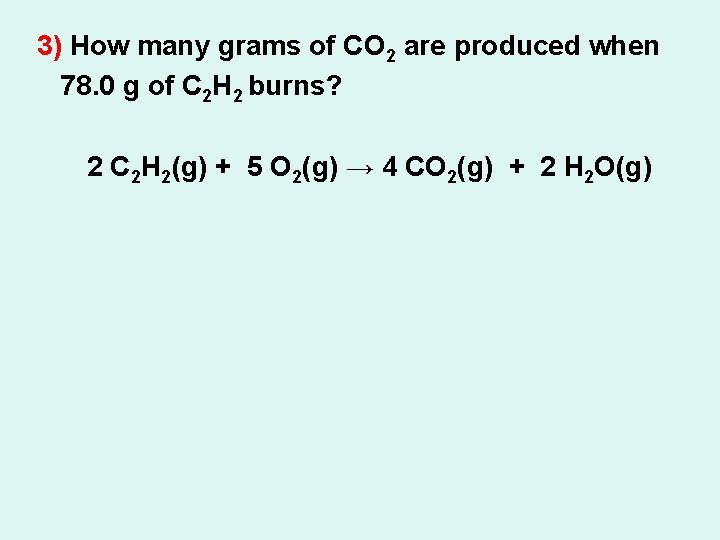
3) How many grams of CO 2 are produced when 78. 0 g of C 2 H 2 burns? 2 C 2 H 2(g) + 5 O 2(g) → 4 CO 2(g) + 2 H 2 O(g)

3) How many grams of CO 2 are produced when 78. 0 g of C 2 H 2 burns? 78. 0 g ? g 2 C 2 H 2(g) + 5 O 2(g) → 4 CO 2(g) + 2 H 2 O(g) 4(44. 01) g 2(26. 04) g = X= = 264 g CO 2 Does it make sense?