Stoichiometry Presentation Chapter 3 Notes 3 5 Determining



- Slides: 9

Stoichiometry Presentation Chapter 3 Notes

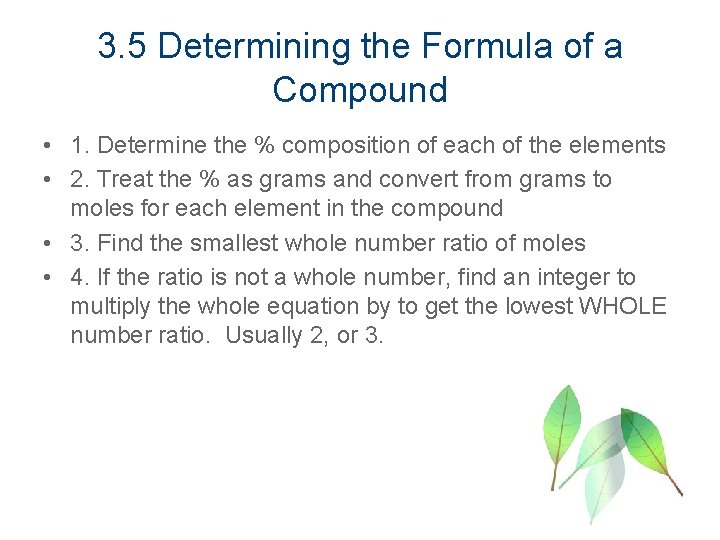
3. 5 Determining the Formula of a Compound • 1. Determine the % composition of each of the elements • 2. Treat the % as grams and convert from grams to moles for each element in the compound • 3. Find the smallest whole number ratio of moles • 4. If the ratio is not a whole number, find an integer to multiply the whole equation by to get the lowest WHOLE number ratio. Usually 2, or 3.

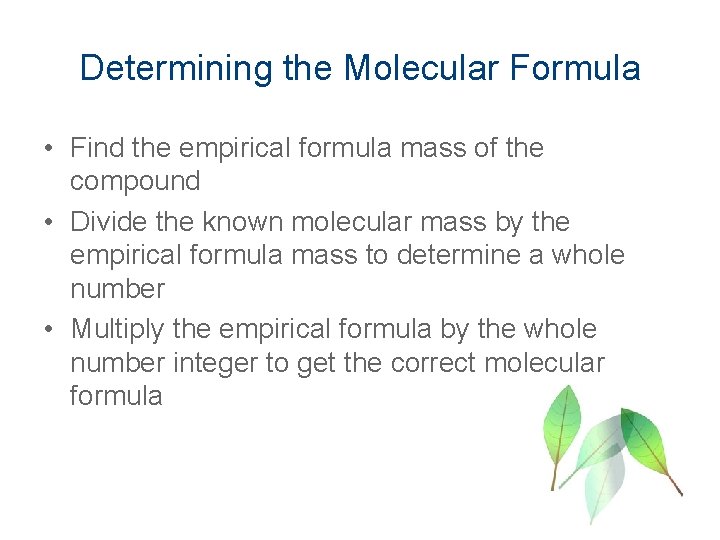
Determining the Molecular Formula • Find the empirical formula mass of the compound • Divide the known molecular mass by the empirical formula mass to determine a whole number • Multiply the empirical formula by the whole number integer to get the correct molecular formula

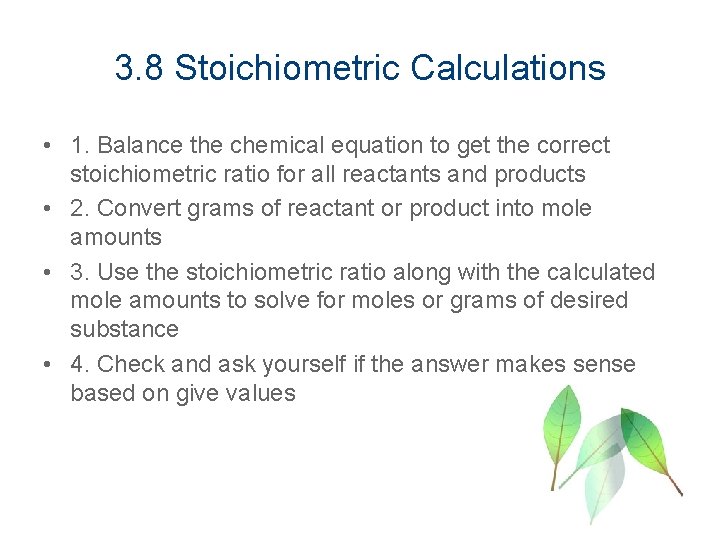
3. 8 Stoichiometric Calculations • 1. Balance the chemical equation to get the correct stoichiometric ratio for all reactants and products • 2. Convert grams of reactant or product into mole amounts • 3. Use the stoichiometric ratio along with the calculated mole amounts to solve for moles or grams of desired substance • 4. Check and ask yourself if the answer makes sense based on give values

3. 9 How to solve a problem with a limiting reagent • 1. Balance the equation to obtain stoichiometric ratios • 2. Convert grams of reactants to moles of reactants • 3. Use the stoichiometric ratios along with the moles of reactants to determine how much product can be obtained using each reactant • 4. The reactant that produces the least amount of product based on given ratios is the LIMITING REAGENT • 5. Once LR is known, we can determine maximum amount of product formed

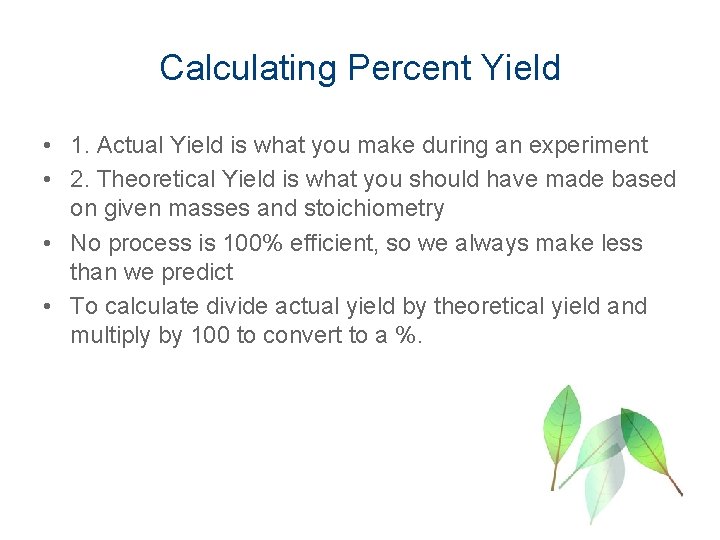
Calculating Percent Yield • 1. Actual Yield is what you make during an experiment • 2. Theoretical Yield is what you should have made based on given masses and stoichiometry • No process is 100% efficient, so we always make less than we predict • To calculate divide actual yield by theoretical yield and multiply by 100 to convert to a %.

3. 10 Combustion Analysis • Process where a sample is burned (Usually a hydrocarbon) in a large excess sample of oxygen gas • Each product is isolated and the weight of each combustion product is determined • Used to calculate the efficiency of a combustion based procedure

How to Calculate • • 1. All elements will be given to you and typically are C, H, O, and N 2. Moles of C will come from amount of CO 2 in liters (22. 4 L = 1 mol) 3. Moles of H will come from amount of H 2 O in grams 4. Oxygen can be figured out once we know the amount of C, and H, and Nitrogen is usually given to us if its part of the compound • 5. Once all mole amounts are known this becomes an empirical formula problem
