Stoichiometry Pick up a book Mole island review
Stoichiometry Pick up a book
Mole island review l Balancing l MINHO chemical equations
Stoichiometry l The mathematics of chemical reactions l Calculate how much product to expect from given reactants l OR figure out how much reactant is needed to produce a desired amount of product

l Look over page 1. l These are 3 Stoich problems worked out. l Put a box around the only NEW part in each problem.
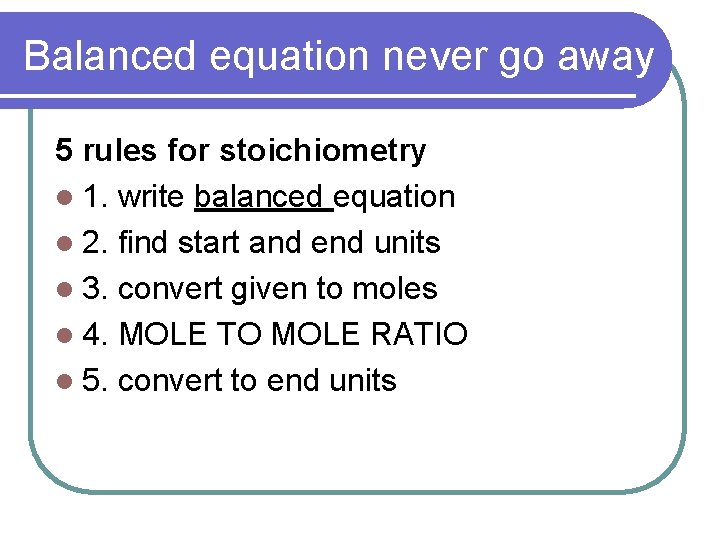
Balanced equation never go away 5 rules for stoichiometry l 1. write balanced equation l 2. find start and end units l 3. convert given to moles l 4. MOLE TO MOLE RATIO l 5. convert to end units
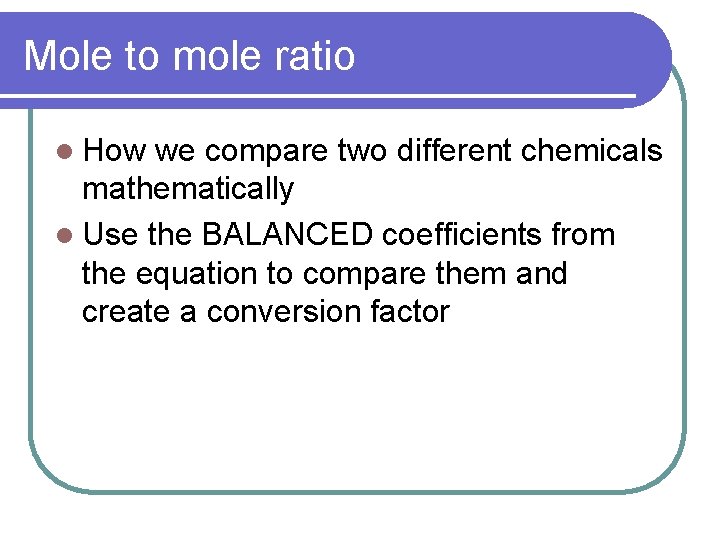
Mole to mole ratio l How we compare two different chemicals mathematically l Use the BALANCED coefficients from the equation to compare them and create a conversion factor
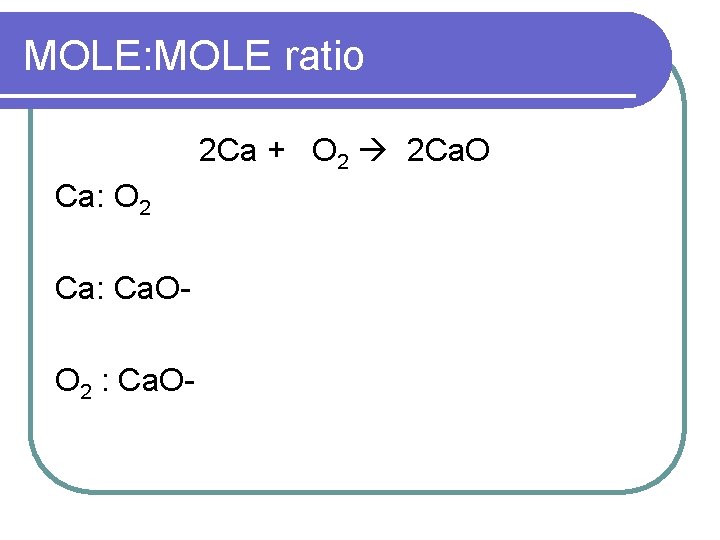
MOLE: MOLE ratio 2 Ca + O 2 2 Ca. O Ca: O 2 Ca: Ca. OO 2 : Ca. O-

Practice alone l Page 2, #1 (a-e)
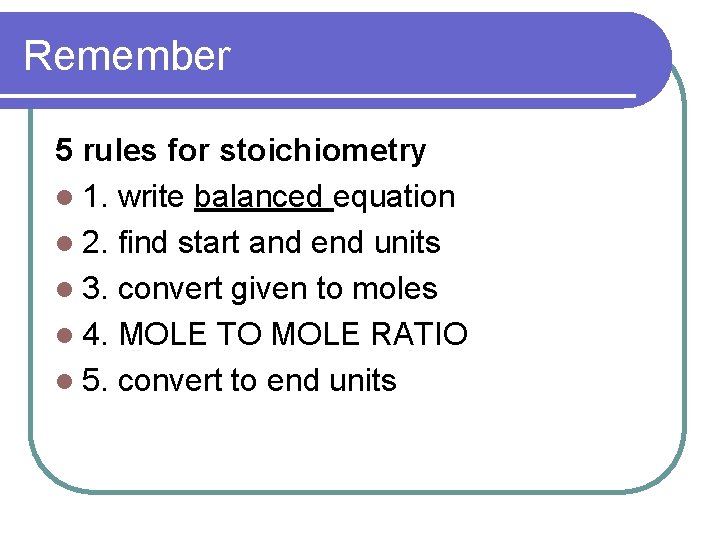
Remember 5 rules for stoichiometry l 1. write balanced equation l 2. find start and end units l 3. convert given to moles l 4. MOLE TO MOLE RATIO l 5. convert to end units
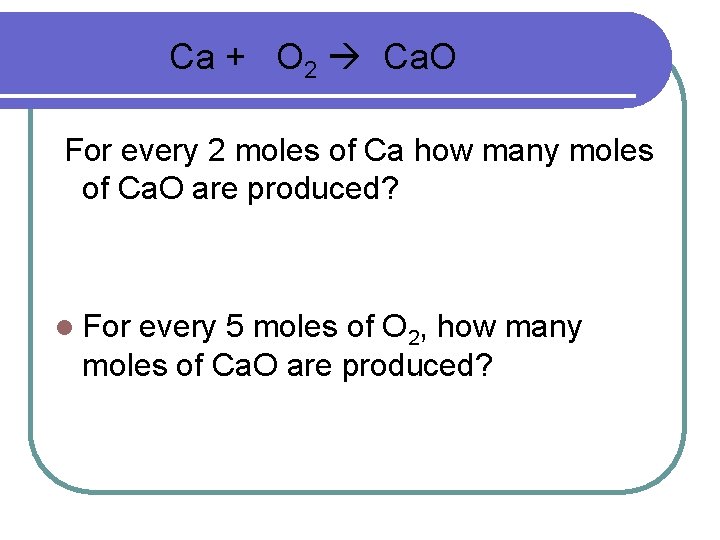
Ca + O 2 Ca. O For every 2 moles of Ca how many moles of Ca. O are produced? l For every 5 moles of O 2, how many moles of Ca. O are produced?

practice l What mass of H 2 can be prepared from the reaction of 1. 50 moles of Zn with HCl?

PRACTICE!! l How many grams of O 2 would be needed to burn 5. 000 g of butane (C 4 H 10)?

Iron in the form of fine wire burns in oxygen to form iron (III) oxide. l 4 Fe + 3 O 2 2 Fe 2 O 3 l How many grams of O 2 are needed to produce 5. 21 grams of Fe 2 O 3? l
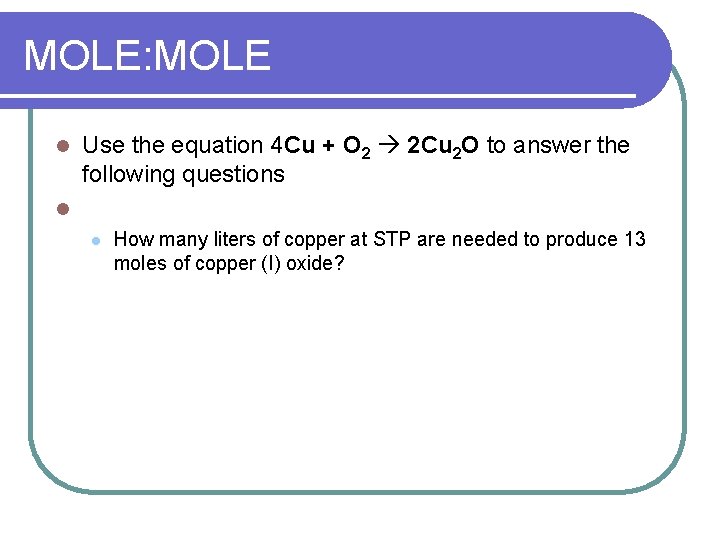
MOLE: MOLE l Use the equation 4 Cu + O 2 2 Cu 2 O to answer the following questions l l How many liters of copper at STP are needed to produce 13 moles of copper (I) oxide?

Classwork l Finish page 2 -3

Practice l Introduction l Page 2 to stoichiometry #2 -3

Homework l Rip out your study guide. l Make sure your name is on it l Staple any separate work to it. l Turn it into the box

Practice l Today we will be working with one other person to complete pages 2 -4 l BOTH PEOPLE MUST SHOW ALL WORK l. I will have an answer key for you to come up and check by when you complete a problem.
Homework Check l Any questions?
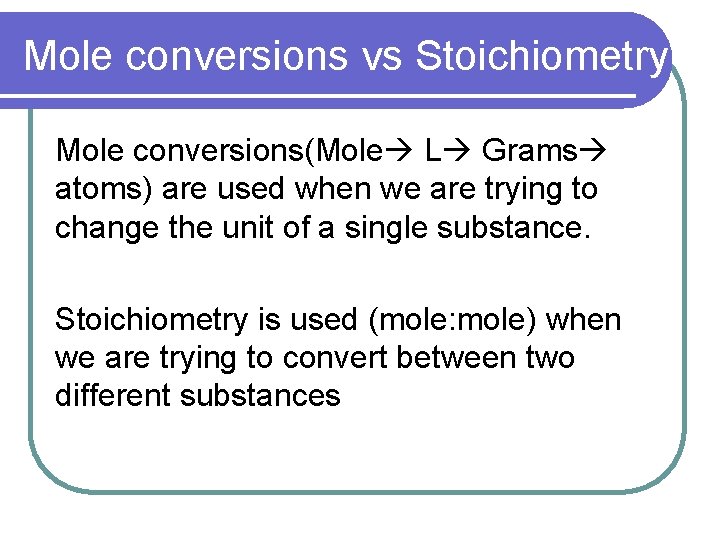
Mole conversions vs Stoichiometry Mole conversions(Mole L Grams atoms) are used when we are trying to change the unit of a single substance. Stoichiometry is used (mole: mole) when we are trying to convert between two different substances

Practice l How many grams are found in 7 moles of Hydrogen gas? l How many atoms are found in 67 grams of CO? l How many liters would 4. 7 x 10 ^23 atoms of oxygen gas make?

Practice Moles and Stoich pg 4 l Worksheet l Get is due by the end of class your problems checked as you go along
- Slides: 22