Stoichiometry Molar Relationships in Chemical Reactions Sections 7
- Slides: 13

Stoichiometry Molar Relationships in Chemical Reactions Sections 7. 1 and 7. 2 - Homework Pg. 320 #1 -9 Pg. 324 #2, 3 Pg. 325 #2, 3, 5 -10

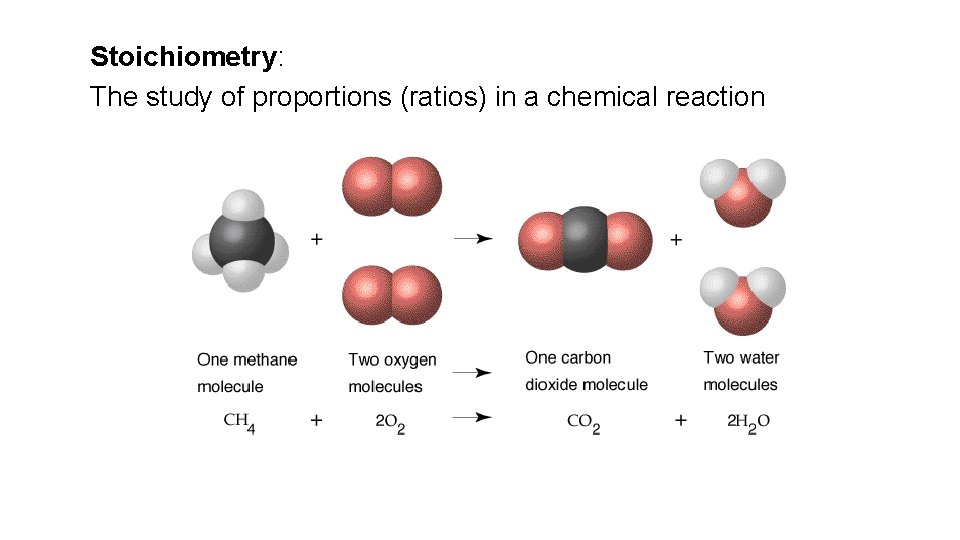
Stoichiometry: The study of proportions (ratios) in a chemical reaction

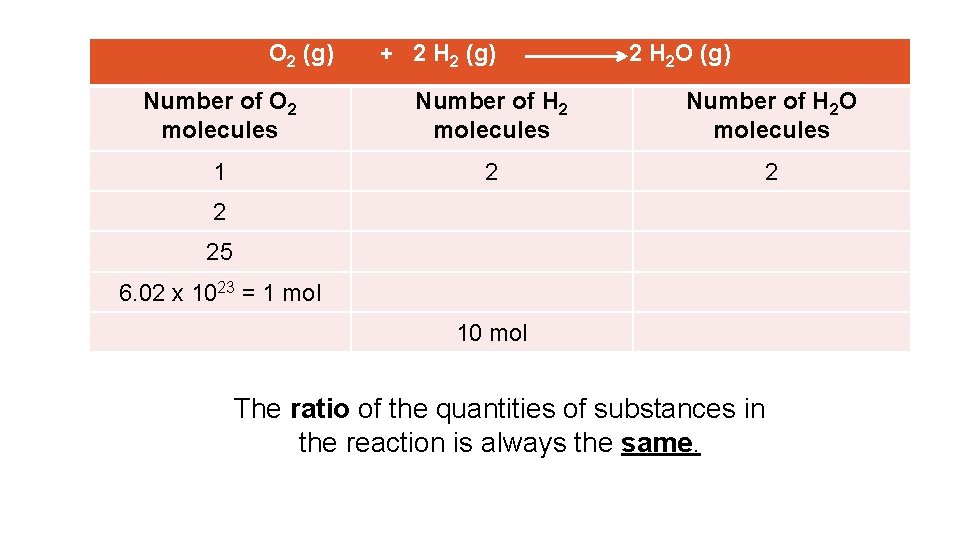
O 2 (g) + 2 H 2 (g) 2 H 2 O (g) Number of O 2 molecules Number of H 2 O molecules 1 2 25 6. 02 x 1023 = 1 mol 10 mol The ratio of the quantities of substances in the reaction is always the same.

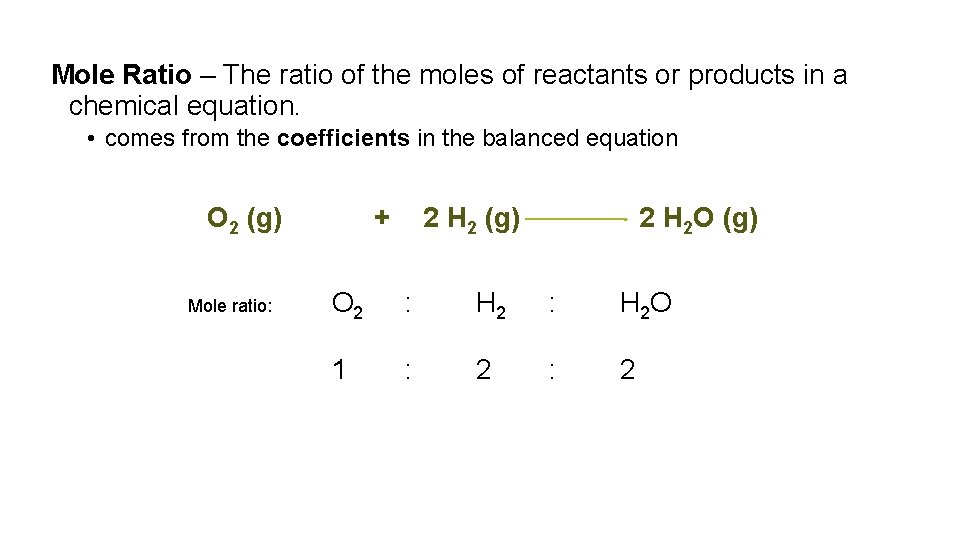
Mole Ratio – The ratio of the moles of reactants or products in a chemical equation. • comes from the coefficients in the balanced equation O 2 (g) Mole ratio: + 2 H 2 (g) 2 H 2 O (g) O 2 : H 2 O 1 : 2

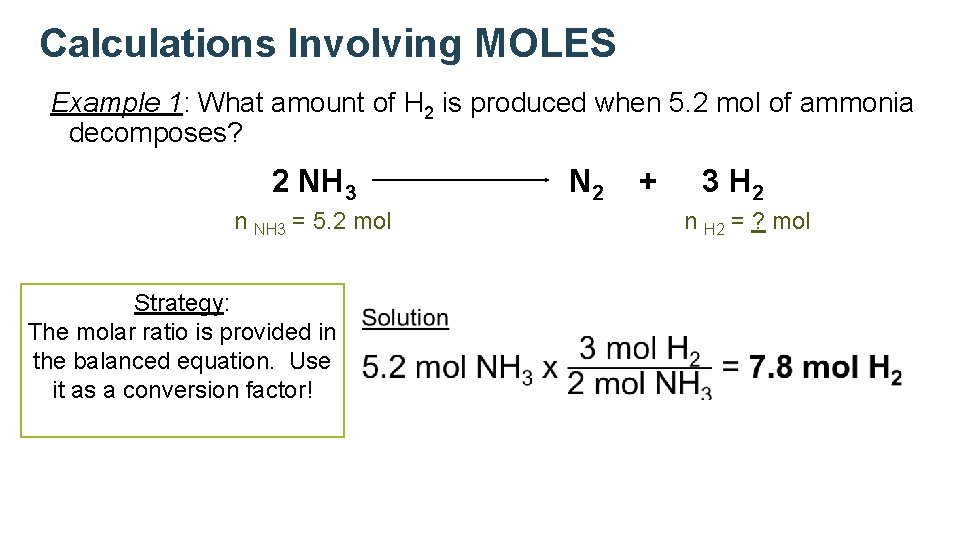
Calculations Involving MOLES Example 1: What amount of H 2 is produced when 5. 2 mol of ammonia decomposes? 2 NH 3 N 2 n NH 3 = 5. 2 mol Strategy: The molar ratio is provided in the balanced equation. Use it as a conversion factor! + 3 H 2 n H 2 = ? mol

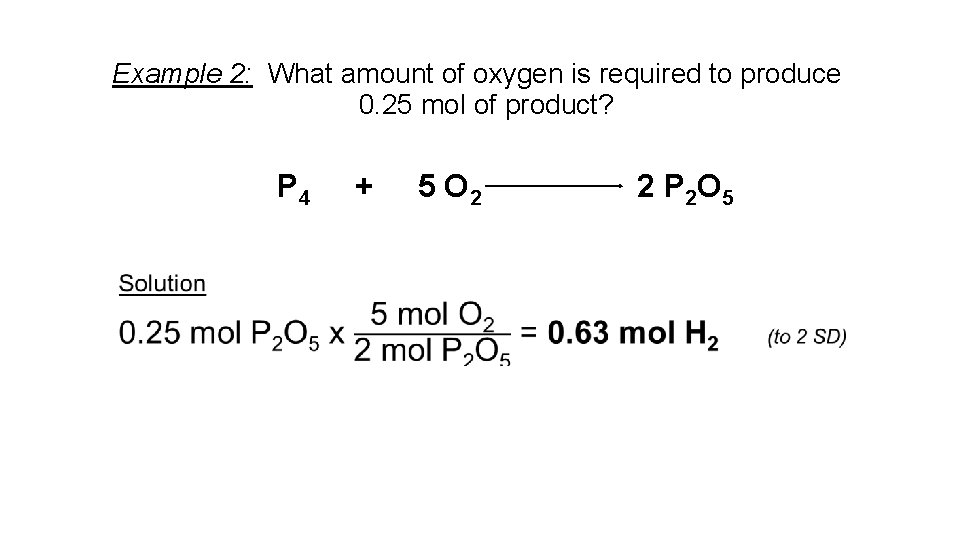
Example 2: What amount of oxygen is required to produce 0. 25 mol of product? P 4 + 5 O 2 2 P 2 O 5

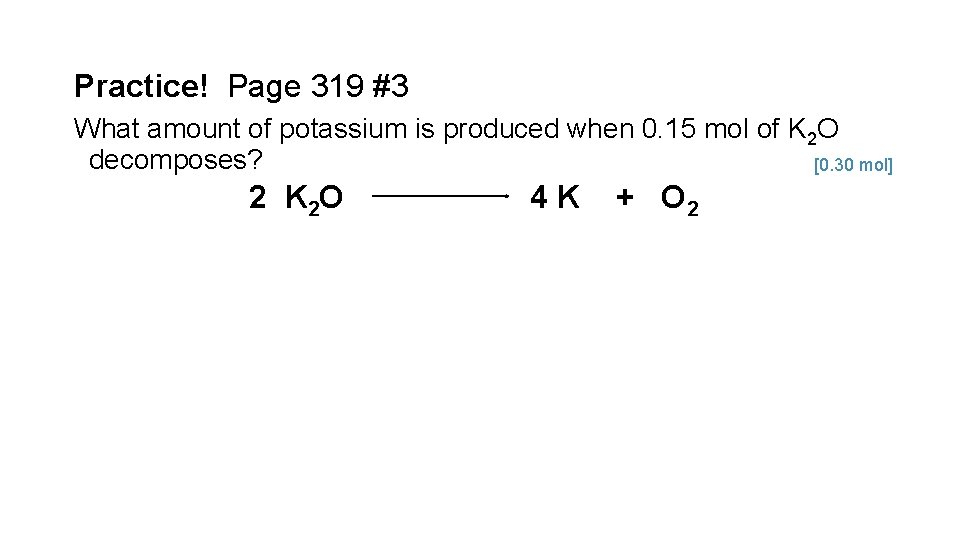
Practice! Page 319 #3 What amount of potassium is produced when 0. 15 mol of K 2 O decomposes? [0. 30 mol] 2 K 2 O 4 K + O 2

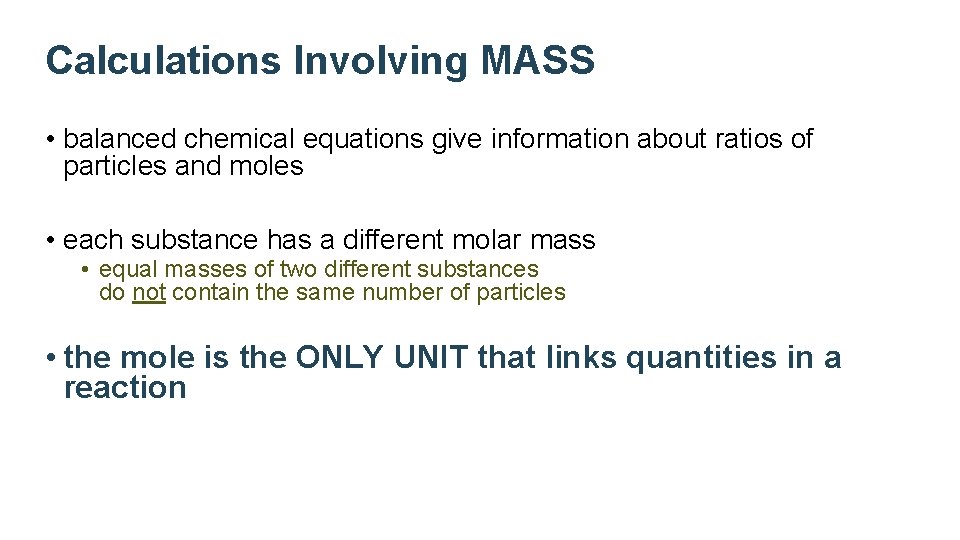
Calculations Involving MASS • balanced chemical equations give information about ratios of particles and moles • each substance has a different molar mass • equal masses of two different substances do not contain the same number of particles • the mole is the ONLY UNIT that links quantities in a reaction

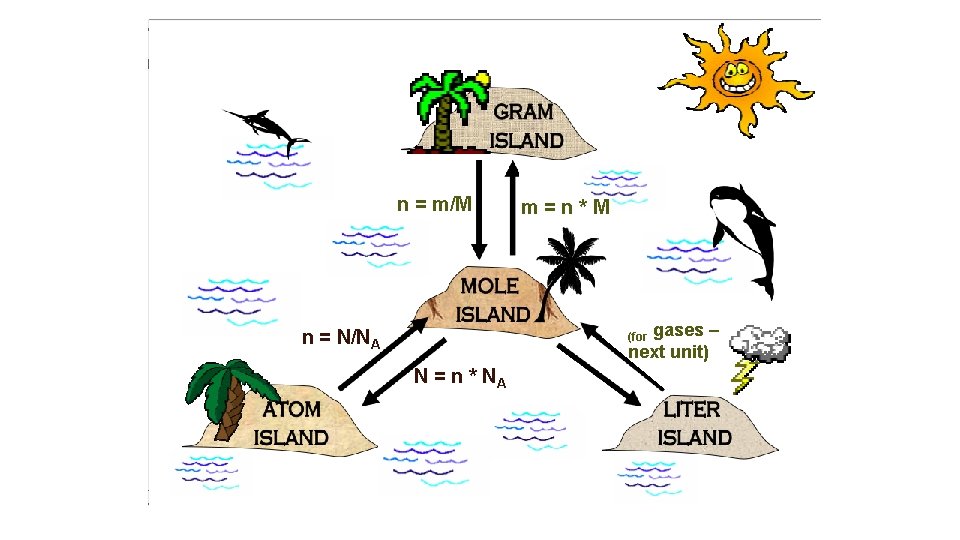
n = m/M m=n*M gases – next unit) n = N/NA (for N = n * NA

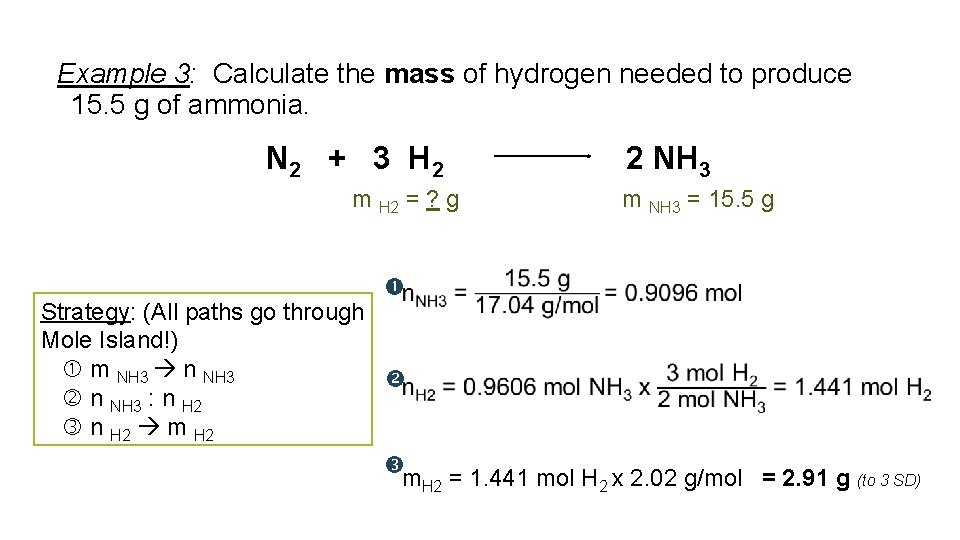
Example 3: Calculate the mass of hydrogen needed to produce 15. 5 g of ammonia. N 2 + 3 H 2 m H 2 = ? g 2 NH 3 m NH 3 = 15. 5 g Strategy: (All paths go through Mole Island!) m NH 3 n NH 3 : n H 2 m. H 2 = 1. 441 mol H 2 x 2. 02 g/mol = 2. 91 g (to 3 SD)

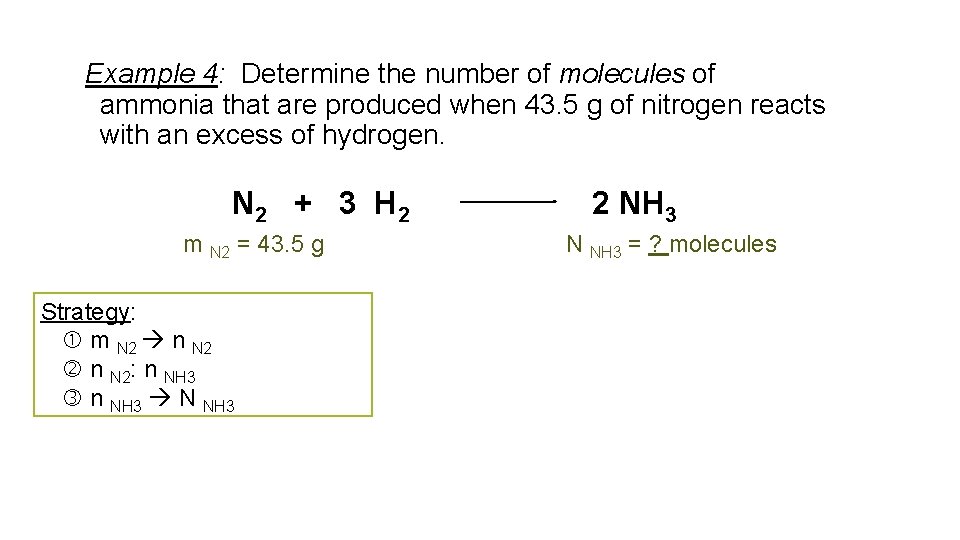
Example 4: Determine the number of molecules of ammonia that are produced when 43. 5 g of nitrogen reacts with an excess of hydrogen. N 2 + 3 H 2 m N 2 = 43. 5 g Strategy: m N 2 n N 2: n NH 3 N NH 3 2 NH 3 N NH 3 = ? molecules

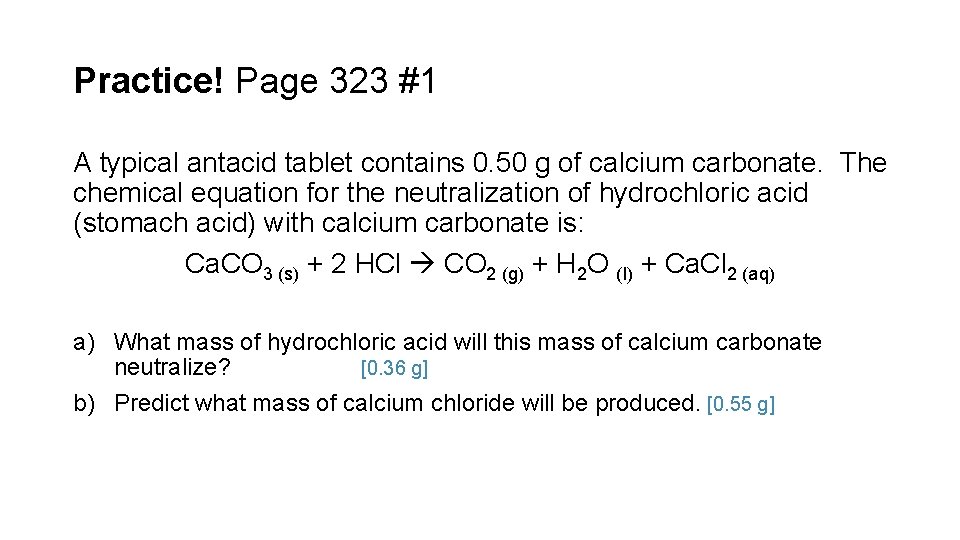
Practice! Page 323 #1 A typical antacid tablet contains 0. 50 g of calcium carbonate. The chemical equation for the neutralization of hydrochloric acid (stomach acid) with calcium carbonate is: Ca. CO 3 (s) + 2 HCl CO 2 (g) + H 2 O (l) + Ca. Cl 2 (aq) a) What mass of hydrochloric acid will this mass of calcium carbonate neutralize? [0. 36 g] b) Predict what mass of calcium chloride will be produced. [0. 55 g]

Homework • Pg. 320 #1 -9 • Pg. 324 #2, 3 • Pg. 325 #2, 3, 5 -10
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Stoichiometry mole island diagram
Stoichiometry mole island diagram Mass relationships in chemical reactions
Mass relationships in chemical reactions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Stoichiometry molar mass
Stoichiometry molar mass Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry How to determine if a single replacement reaction occurs
How to determine if a single replacement reaction occurs Examples of redox reaction
Examples of redox reaction

























